JEE Advance - Chemistry (2024 - Paper 1 Online - No. 12)
In the following reaction sequence, the major product $\mathbf{P}$ is formed.

Glycerol reacts completely with excess $\mathbf{P}$ in the presence of an acid catalyst to form $\mathbf{Q}$. Reaction of $\mathbf{Q}$ with excess $\mathrm{NaOH}$ followed by the treatment with $\mathrm{CaCl}_2$ yields Ca-soap $\mathbf{R}$, quantitatively. Starting with one mole of $\mathbf{Q}$, the amount of $\mathbf{R}$ produced in gram is __________.
[Given, atomic weight: $\mathrm{H}=1, \mathrm{C}=12, \mathrm{~N}=14, \mathrm{O}=16, \mathrm{Na}=23, \mathrm{Cl}=35, \mathrm{Ca}=40$ ]
Answer
909
Explanation
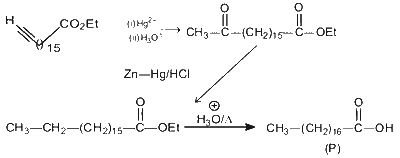
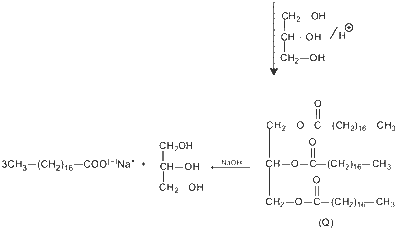
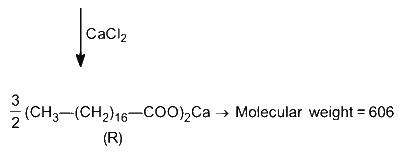
$\therefore 1$ mole $Q$ produce $\frac{3}{2}$ moles $R$
$\therefore$ Mass of $R$ produce $=\frac{3}{2} \times 606=909 \mathrm{~g}$
Comments (0)


