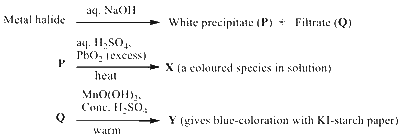
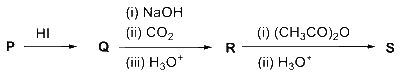
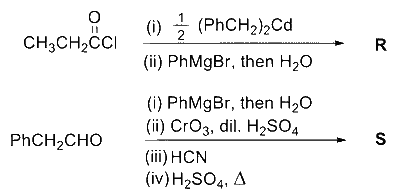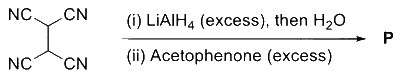JEE Advance - Chemistry (2023 - Paper 1 Online)
5
Plotting $1 / \Lambda_{\mathrm{m}}$ against $\mathrm{c} \Lambda_{\mathrm{m}}$ for aqueous solutions of a monobasic weak acid $(\mathrm{HX})$ resulted in a straight line with $\mathrm{y}$-axis intercept of $\mathrm{P}$ and slope of $\mathrm{S}$. The ratio $\mathrm{P} / \mathrm{S}$ is
$$ \begin{aligned} & {\left[\Lambda_{\mathrm{m}}=\right.\text { molar conductivity }} \\\\ & \Lambda_{\mathrm{m}}^{\mathrm{o}}=\text { limiting molar conductivity } \\\\ & \mathrm{c}=\text { molar concentration } \\\\ & \left.\mathrm{K}_{\mathrm{a}}=\text { dissociation constant of } \mathrm{HX}\right] \end{aligned} $$
$$ \begin{aligned} & {\left[\Lambda_{\mathrm{m}}=\right.\text { molar conductivity }} \\\\ & \Lambda_{\mathrm{m}}^{\mathrm{o}}=\text { limiting molar conductivity } \\\\ & \mathrm{c}=\text { molar concentration } \\\\ & \left.\mathrm{K}_{\mathrm{a}}=\text { dissociation constant of } \mathrm{HX}\right] \end{aligned} $$
Answer
(A)
$\mathrm{K}_{\mathrm{a}} \Lambda_{\mathrm{m}}^{\mathrm{o}}$
8
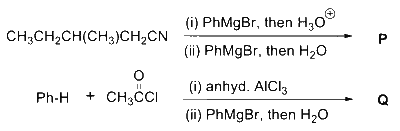
The stoichiometric reaction of $516 \mathrm{~g}$ of dimethyldichlorosilane with water results in a tetrameric cyclic product $\mathbf{X}$ in $75 \%$ yield. The weight (in g) of $\mathbf{X}$ obtained is _______.
[Use, molar mass $\left(\mathrm{g} ~\mathrm{mol}^{-1}\right): \mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{Si}=28, \mathrm{Cl}=35.5$ ]
[Use, molar mass $\left(\mathrm{g} ~\mathrm{mol}^{-1}\right): \mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{Si}=28, \mathrm{Cl}=35.5$ ]
Answer
222
9
A gas has a compressibility factor of 0.5 and a molar volume of $0.4 ~\mathrm{dm}^3 \mathrm{~mol}^{-1}$ at a temperature of $800 \mathrm{~K}$ and pressure $\mathbf{x}$ atm. If it shows ideal gas behaviour at the same temperature and pressure, the molar volume will be $\mathbf{y} ~\mathrm{dm}^3 \mathrm{~mol}^{-1}$. The value of $\mathbf{x} / \mathbf{y}$ is __________.
[Use: Gas constant, $\mathrm{R}=8 \times 10^{-2} \mathrm{~L}$ atm $\mathrm{K}^{-1} \mathrm{~mol}^{-1}$ ]
[Use: Gas constant, $\mathrm{R}=8 \times 10^{-2} \mathrm{~L}$ atm $\mathrm{K}^{-1} \mathrm{~mol}^{-1}$ ]
Answer
100
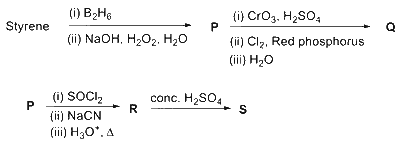
10
The plot of $\log k_f$ versus $1 / T$ for a reversible reaction $\mathrm{A}(\mathrm{g}) \rightleftharpoons \mathrm{P}(\mathrm{g})$ is shown.
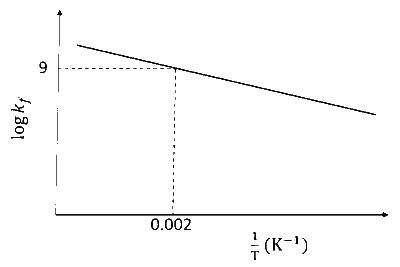
Pre-exponential factors for the forward and backward reactions are $10^{15} \mathrm{~s}^{-1}$ and $10^{11} \mathrm{~s}^{-1}$, respectively. If the value of $\log K$ for the reaction at $500 \mathrm{~K}$ is 6 , the value of $\left|\log k_b\right|$ at $250 \mathrm{~K}$ is ______.
$$ \begin{aligned} & {[K=\text { equilibrium constant of the reaction }} \\\\ & k_f=\text { rate constant of forward reaction } \\\\ & \left.k_b=\text { rate constant of backward reaction }\right] \end{aligned} $$

Pre-exponential factors for the forward and backward reactions are $10^{15} \mathrm{~s}^{-1}$ and $10^{11} \mathrm{~s}^{-1}$, respectively. If the value of $\log K$ for the reaction at $500 \mathrm{~K}$ is 6 , the value of $\left|\log k_b\right|$ at $250 \mathrm{~K}$ is ______.
$$ \begin{aligned} & {[K=\text { equilibrium constant of the reaction }} \\\\ & k_f=\text { rate constant of forward reaction } \\\\ & \left.k_b=\text { rate constant of backward reaction }\right] \end{aligned} $$
Answer
5
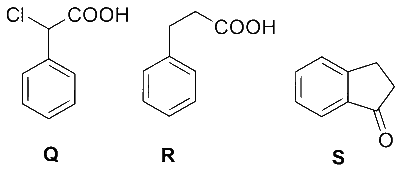
11
One mole of an ideal monoatomic gas undergoes two reversible processes $(\mathrm{A} \rightarrow \mathrm{B}$ and $\mathrm{B} \rightarrow \mathrm{C})$ as shown in the given figure:
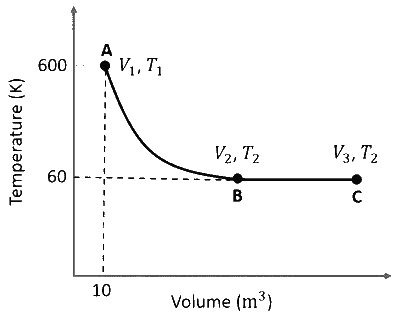
$\mathrm{A} \rightarrow \mathrm{B}$ is an adiabatic process. If the total heat absorbed in the entire process $(\mathrm{A} \rightarrow \mathrm{B}$ and $\mathrm{B} \rightarrow \mathrm{C})$ is $\mathrm{R} T_2 \ln 10$, the value of $2 \log V_3$ is _______.
[Use, molar heat capacity of the gas at constant pressure, $C_{\mathrm{p}, \mathrm{m}}=\frac{5}{2} \mathrm{R}$ ]

$\mathrm{A} \rightarrow \mathrm{B}$ is an adiabatic process. If the total heat absorbed in the entire process $(\mathrm{A} \rightarrow \mathrm{B}$ and $\mathrm{B} \rightarrow \mathrm{C})$ is $\mathrm{R} T_2 \ln 10$, the value of $2 \log V_3$ is _______.
[Use, molar heat capacity of the gas at constant pressure, $C_{\mathrm{p}, \mathrm{m}}=\frac{5}{2} \mathrm{R}$ ]
Answer
7
12
In a one-litre flask, 6 moles of $A$ undergoes the reaction $A(\mathrm{~g}) \rightleftharpoons P(\mathrm{~g})$. The progress of product formation at two temperatures (in Kelvin), $\mathrm{T}_1$ and $\mathrm{T}_2$, is shown in the figure:
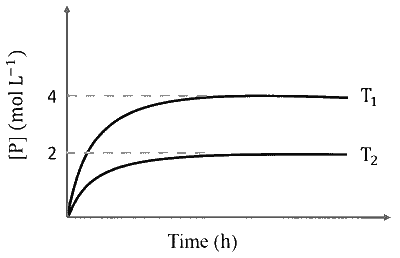
If $\mathrm{T}_1=2 \mathrm{~T}_2$ and $\left(\Delta \mathrm{G}_2^{\Theta}-\Delta \mathrm{G}_1^{\Theta}\right)=\mathrm{RT}_2 \ln \mathrm{x}$, then the value of $\mathrm{x}$ is _______.
$\left[\Delta \mathrm{G}_1^{\Theta}\right.$ and $\Delta \mathrm{G}_2^{\Theta}$ are standard Gibb's free energy change for the reaction at temperatures $\mathrm{T}_1$ and $\mathrm{T}_2$, respectively.]

If $\mathrm{T}_1=2 \mathrm{~T}_2$ and $\left(\Delta \mathrm{G}_2^{\Theta}-\Delta \mathrm{G}_1^{\Theta}\right)=\mathrm{RT}_2 \ln \mathrm{x}$, then the value of $\mathrm{x}$ is _______.
$\left[\Delta \mathrm{G}_1^{\Theta}\right.$ and $\Delta \mathrm{G}_2^{\Theta}$ are standard Gibb's free energy change for the reaction at temperatures $\mathrm{T}_1$ and $\mathrm{T}_2$, respectively.]
Answer
8
14
Match the reactions (in the given stoichiometry of the reactants) in List-I with one of their products given in List-II and choose the correct option.
| List - I | List - II |
|---|---|
| (P) $\mathrm{P}_2 \mathrm{O}_3+3 \mathrm{H}_2 \mathrm{O} \rightarrow$ | (1) $\mathrm{P}(\mathrm{O})\left(\mathrm{OCH}_3\right) \mathrm{Cl}_2$ |
| (Q) $\mathrm{P}_4+3 \mathrm{NaOH}+3 \mathrm{H}_2 \mathrm{O} \rightarrow$ | (2) $\mathrm{H}_3 \mathrm{PO}_3$ |
| (R) $\mathrm{PCl}_5+\mathrm{CH}_3 \mathrm{COOH} \rightarrow$ | (3) $\mathrm{PH}_3$ |
| (S) $\mathrm{H}_3 \mathrm{PO}_2+2 \mathrm{H}_2 \mathrm{O}+4 \mathrm{AgNO}_3 \rightarrow$ | (4) $\mathrm{POCl}_3$ |
| (5) $\mathrm{H}_3 \mathrm{PO}_4$ |
Answer
(D)
$\mathrm{P} \rightarrow 2 ; \mathrm{Q} \rightarrow 3 ; \mathrm{R} \rightarrow 4 ; \mathrm{S} \rightarrow 5$
15
Match the electronic configurations in List-I with appropriate metal complex ions in List-II and choose the correct option.
[Atomic Number: $\mathrm{Fe}=26, \mathrm{Mn}=25, \mathrm{Co}=27$ ]
[Atomic Number: $\mathrm{Fe}=26, \mathrm{Mn}=25, \mathrm{Co}=27$ ]
| List - I | List - II |
|---|---|
| (P) $t_{2 g}^6 e_g^0$ | (1) $\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ |
| (Q) $t_{2 g}^3 e_g^2$ | (2) $\left[\mathrm{Mn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ |
| (R) $\mathrm{e}^2 \mathrm{t}_2^3$ | (3) $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}$ |
| (S) $t_{2 g}^4 e_g^2$ | (4) $\left[\mathrm{FeCl}_4\right]^{-}$ |
| (5) $\left[\mathrm{CoCl}_4\right]^{2-}$ |
Answer
(D)
$\mathrm{P} \rightarrow 3 ; \mathrm{Q} \rightarrow 2 ; \mathrm{R} \rightarrow 4 ; \mathrm{S} \rightarrow 1$
16
Match the reactions in List-I with the features of their products in List-II and choose the correct option.
| List - I | List - II |
|---|---|
(P) 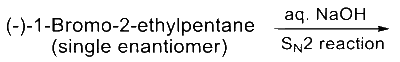 |
(1) Inversion of configuration |
(Q)  |
(2) Retention of configuration |
(R)  |
(3) Mixture of enantiomers |
(S)  |
(4) Mixture of structural isomers |
| (5) Mixture of diastereomers |
Answer
(B)
$\mathrm{P} \rightarrow 2 ; \mathrm{Q} \rightarrow 1 ; \mathrm{R} \rightarrow 3 ; \mathrm{S} \rightarrow 5$
17
The major products obtained from the reactions in List-II are the reactants for the named reactions mentioned in List-I. Match List-I with List-II and choose the correct option.
| List - I | List - II |
|---|---|
| (P) Etard reaction | (1) Acetophenone $\stackrel{\mathrm{Zn}-\mathrm{Hg}, \mathrm{HCl}}{\longrightarrow}$ |
| (Q) Gattermann reaction | (2) $$ \text { Toluene } \underset{\text { (ii) } \mathrm{SOCl}_2}{\stackrel{\text { (i) } \mathrm{KMnO}_4, \mathrm{KOH}, \Delta}{\longrightarrow}} $$ |
| (R) Gattermann-Koch reaction | (3) $$ \text { Benzene } \underset{\text { anhyd. } \mathrm{AlCl}_3}{\stackrel{\mathrm{CH}_3 \mathrm{Cl}}{\longrightarrow}} $$ |
| (S) Rosenmund reduction | (4) $$ \text { Aniline } \underset{273-278 \mathrm{~K}}{\stackrel{\mathrm{NaNO}_2 / \mathrm{HCl}}{\longrightarrow}} $$ |
| (5) $$ \text { Phenol } \stackrel{\mathrm{Zn}, \Delta}{\longrightarrow} $$ |
Answer
(D)
$$
\mathrm{P} \rightarrow 3 ; \mathrm{Q} \rightarrow 4 ; \mathrm{R} \rightarrow 5 ; \mathrm{S} \rightarrow 2
$$