JEE Advance - Chemistry (2023 - Paper 1 Online - No. 15)
Match the electronic configurations in List-I with appropriate metal complex ions in List-II and choose the correct option.
[Atomic Number: $\mathrm{Fe}=26, \mathrm{Mn}=25, \mathrm{Co}=27$ ]
[Atomic Number: $\mathrm{Fe}=26, \mathrm{Mn}=25, \mathrm{Co}=27$ ]
| List - I | List - II |
|---|---|
| (P) $t_{2 g}^6 e_g^0$ | (1) $\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ |
| (Q) $t_{2 g}^3 e_g^2$ | (2) $\left[\mathrm{Mn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ |
| (R) $\mathrm{e}^2 \mathrm{t}_2^3$ | (3) $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}$ |
| (S) $t_{2 g}^4 e_g^2$ | (4) $\left[\mathrm{FeCl}_4\right]^{-}$ |
| (5) $\left[\mathrm{CoCl}_4\right]^{2-}$ |
$\mathrm{P} \rightarrow 1 ; \mathrm{Q} \rightarrow 4 ; \mathrm{R} \rightarrow 2 ; \mathrm{S} \rightarrow 3$
$P \rightarrow 1 ; \mathrm{Q} \rightarrow 2 ; \mathrm{R} \rightarrow 4 ; \mathrm{S} \rightarrow 5$
$\mathrm{P} \rightarrow 3 ; \mathrm{Q} \rightarrow 2 ; \mathrm{R} \rightarrow 5 ; \mathrm{S} \rightarrow 1$
$\mathrm{P} \rightarrow 3 ; \mathrm{Q} \rightarrow 2 ; \mathrm{R} \rightarrow 4 ; \mathrm{S} \rightarrow 1$
Explanation
1.
$$ \begin{aligned} & {\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}} \\\\ & \mathrm{Fe}(26) \longrightarrow 3 \mathrm{~d}^6 4 \mathrm{~s}^2 \\\\ & \mathrm{Fe}^{2+} \longrightarrow 3 \mathrm{~d}^6, \mathrm{H}_2 \mathrm{O} \text { is a weak field ligand. } \end{aligned} $$
So, the pairing does not take place.
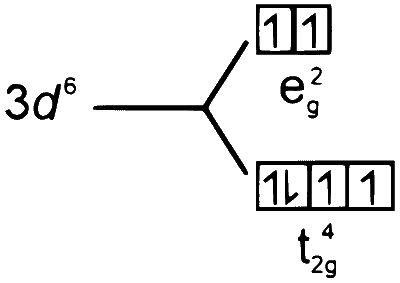
2.
$$ \begin{aligned} & {\left[\mathrm{Mn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}} \\\\ & \mathrm{Mn}(25) \longrightarrow 3 \mathrm{~d}^5 4 \mathrm{~s}^2 \\\\ & \mathrm{Mn}^{2+} \longrightarrow 3 \mathrm{~d}^5, \mathrm{H}_2 \mathrm{O} \text { is a weak field ligand. } \end{aligned} $$
So, the pairing does not take place.
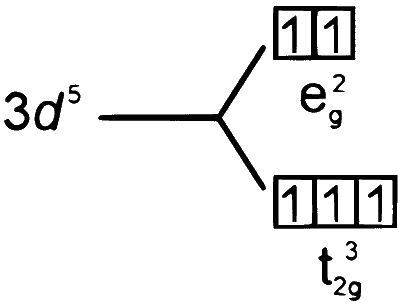
3.
$\left[\mathrm{CO}\left(\mathrm{NH}_3\right)_6\right]^{3+}$
$$ \mathrm{CO}^{3+} \longrightarrow[\mathrm{Ar}] 3 \mathrm{~d}^6 $$
$\mathrm{NH}_3$ is a strong field ligand. So, the pairing takes place.
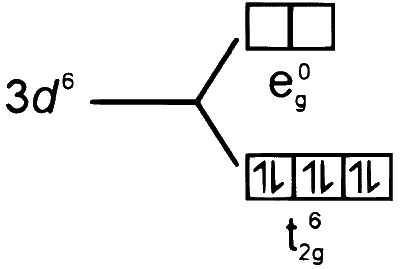
4.
$$ \begin{aligned} & \begin{aligned} {\left[\mathrm{FeCl}_4\right]^{-} } \end{aligned} \\\\ & x+4(-1)=-1 \\\\ & x=+3 \\\\ & \mathrm{Fe}(26) \longrightarrow 3 \mathrm{~d}^6 4 \mathrm{~s}^2 \\\\ & \mathrm{Fe}^{+3} \longrightarrow[\mathrm{Ar}] 3 \mathrm{~d}^5 \end{aligned} $$
$\mathrm{FeCl}_4$ is tetrahedral complex.
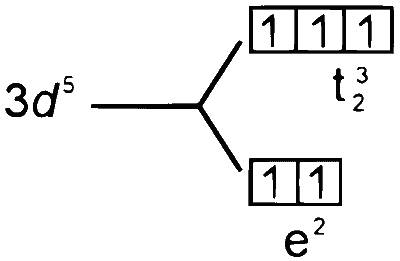
$\begin{aligned} & \text { 5. }\left[\mathrm{COCl}_4\right]^{2-} \\\\ & \mathrm{CO} \longrightarrow 3 \mathrm{~d}^7 4 \mathrm{~s}^2 \\\\ & \mathrm{CO}^{2+} \longrightarrow 3 \mathrm{~d}^7 \end{aligned}$
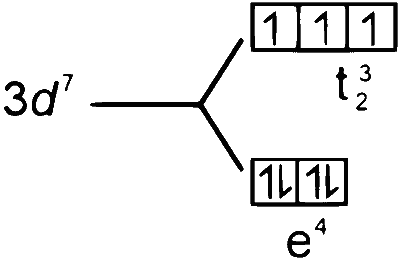
$\therefore \mathrm{P} \rightarrow 3, \mathrm{Q} \rightarrow 2, \mathrm{R} \rightarrow 4, \mathrm{~S} \rightarrow 1$
$$ \begin{aligned} & {\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}} \\\\ & \mathrm{Fe}(26) \longrightarrow 3 \mathrm{~d}^6 4 \mathrm{~s}^2 \\\\ & \mathrm{Fe}^{2+} \longrightarrow 3 \mathrm{~d}^6, \mathrm{H}_2 \mathrm{O} \text { is a weak field ligand. } \end{aligned} $$
So, the pairing does not take place.

2.
$$ \begin{aligned} & {\left[\mathrm{Mn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}} \\\\ & \mathrm{Mn}(25) \longrightarrow 3 \mathrm{~d}^5 4 \mathrm{~s}^2 \\\\ & \mathrm{Mn}^{2+} \longrightarrow 3 \mathrm{~d}^5, \mathrm{H}_2 \mathrm{O} \text { is a weak field ligand. } \end{aligned} $$
So, the pairing does not take place.

3.
$\left[\mathrm{CO}\left(\mathrm{NH}_3\right)_6\right]^{3+}$
$$ \mathrm{CO}^{3+} \longrightarrow[\mathrm{Ar}] 3 \mathrm{~d}^6 $$
$\mathrm{NH}_3$ is a strong field ligand. So, the pairing takes place.

4.
$$ \begin{aligned} & \begin{aligned} {\left[\mathrm{FeCl}_4\right]^{-} } \end{aligned} \\\\ & x+4(-1)=-1 \\\\ & x=+3 \\\\ & \mathrm{Fe}(26) \longrightarrow 3 \mathrm{~d}^6 4 \mathrm{~s}^2 \\\\ & \mathrm{Fe}^{+3} \longrightarrow[\mathrm{Ar}] 3 \mathrm{~d}^5 \end{aligned} $$
$\mathrm{FeCl}_4$ is tetrahedral complex.

$\begin{aligned} & \text { 5. }\left[\mathrm{COCl}_4\right]^{2-} \\\\ & \mathrm{CO} \longrightarrow 3 \mathrm{~d}^7 4 \mathrm{~s}^2 \\\\ & \mathrm{CO}^{2+} \longrightarrow 3 \mathrm{~d}^7 \end{aligned}$

$\therefore \mathrm{P} \rightarrow 3, \mathrm{Q} \rightarrow 2, \mathrm{R} \rightarrow 4, \mathrm{~S} \rightarrow 1$
Comments (0)


