JEE Advance - Chemistry (2023 - Paper 1 Online - No. 8)
The stoichiometric reaction of $516 \mathrm{~g}$ of dimethyldichlorosilane with water results in a tetrameric cyclic product $\mathbf{X}$ in $75 \%$ yield. The weight (in g) of $\mathbf{X}$ obtained is _______.
[Use, molar mass $\left(\mathrm{g} ~\mathrm{mol}^{-1}\right): \mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{Si}=28, \mathrm{Cl}=35.5$ ]
[Use, molar mass $\left(\mathrm{g} ~\mathrm{mol}^{-1}\right): \mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{Si}=28, \mathrm{Cl}=35.5$ ]
Answer
222
Explanation
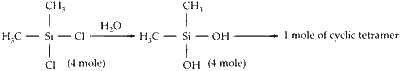
No. of moles $=\frac{\text { Given mass }}{\text { Molar mass }}=\frac{516}{129}=4$
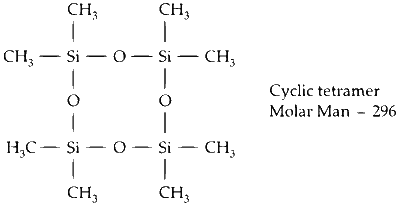
$\begin{aligned} & \therefore \text { Percentage yield }=\frac{75}{100}=0.75 \\\\ & \therefore \text { Mole formed of cyclic tetramer }=0.75 \\\\ & \therefore \text { Weight }=0.75 \times 296=222 \mathrm{~g}\end{aligned}$
Comments (0)


