JEE Advance - Chemistry (2023 - Paper 1 Online - No. 4)
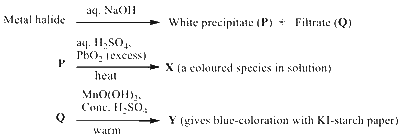
In the scheme given below, $\mathbf{X}$ and $\mathbf{Y}$, respectively, are

$\mathrm{CrO}_4{ }^{2-}$ and $\mathrm{Br}_2$
$\mathrm{MnO}_4{ }^{2-}$ and $\mathrm{Cl}_2$
$\mathrm{MnO}_4{ }^{-}$and $\mathrm{Cl}_2$
$\mathrm{MnSO}_4$ and $\mathrm{HOCl}$
Explanation
$$
\mathrm{MnCl}_2 \stackrel{\mathrm{NaOH}(\mathrm{aq})}{\longrightarrow} \underset{(\mathrm{P})}{\mathrm{Mn}\left(\mathrm{OH}_2\right)}+\underset{(\mathrm{Q})}{\mathrm{NaCl}}
$$
$$ \mathrm{Mn}(\mathrm{OH})_2 \underset{\mathrm{PbO}_2(\text { excess })}{\stackrel{\text { aq. } \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow}} \underset{(\mathrm{X})}{\mathrm{MnO}_4^{-}}+\mathrm{Pb}^{2+} $$
$$ \mathrm{NaCl} \frac{\mathrm{MnO}(\mathrm{OH})_2}{\text { conc. } \mathrm{H}_2 \mathrm{SO}_4 \text { warm }} \underset{(\mathrm{Y})}{\mathrm{Cl}_2} $$
$$ \mathrm{Mn}(\mathrm{OH})_2 \underset{\mathrm{PbO}_2(\text { excess })}{\stackrel{\text { aq. } \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow}} \underset{(\mathrm{X})}{\mathrm{MnO}_4^{-}}+\mathrm{Pb}^{2+} $$
$$ \mathrm{NaCl} \frac{\mathrm{MnO}(\mathrm{OH})_2}{\text { conc. } \mathrm{H}_2 \mathrm{SO}_4 \text { warm }} \underset{(\mathrm{Y})}{\mathrm{Cl}_2} $$
Comments (0)


