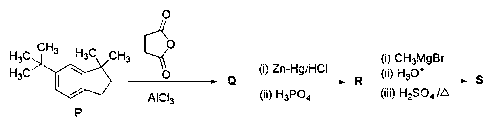
JEE Advance - Chemistry (2020 - Paper 1 Offline)
3
A colourless aqueous solution contains nitrates of two metals, X and Y. When it was added to an aqueous solution of NaCl, a white precipitate was formed. This precipitate was found to be partly soluble in hot water to give a residue P and a solution Q. The residue P was soluble in aqueous NH3 and also in excess sodium thiosulphate. The hot solution Q gave a yellow precipitate with KI. The metals X and Y, respectively, are
Answer
(A)
Ag and Pb
13
5.00 mL of 0.10 M oxalic acid solution taken in a conical flask is titrated against NaOH from a burette using phenolphthalein indicator. The volume of NaOH required for the appearance of permanent faint pink color is tabulated below for five experiments. What is the concentration, in molarity, of the NaOH solution?
| Exp. No. | Vol. of NaOH (mL) |
|---|---|
| 1 | 12.5 |
| 2 | 10.5 |
| 3 | 9.0 |
| 4 | 9.0 |
| 5 | 9.0 |
Answer
0.11
14
Consider the reaction,
A $$\rightleftharpoons $$ B
at 1000 K. At time t', the temperature of the system was increased to 2000 K and the system was allowed to reach equilibrium. Throughout this experiment the partial pressure of A was maintained at 1 bar. Given, below is the plot of the partial pressure of B with time. What is the ratio of the standard Gibbs energy of the reaction at 1000 K to that at 2000 K?
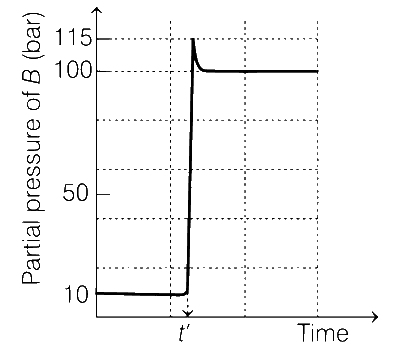
A $$\rightleftharpoons $$ B
at 1000 K. At time t', the temperature of the system was increased to 2000 K and the system was allowed to reach equilibrium. Throughout this experiment the partial pressure of A was maintained at 1 bar. Given, below is the plot of the partial pressure of B with time. What is the ratio of the standard Gibbs energy of the reaction at 1000 K to that at 2000 K?

Answer
0.25
15
Consider a 70% efficient hydrogen-oxygen fuel cell working under standard conditions at 1 bar and 298 K. Its cell reaction is
$${H_2}(g) + {1 \over 2}{O_2}(g)\buildrel {} \over \longrightarrow {H_2}O(l)$$
The work derived from the cell on the consumption of 1.0 $$ \times $$ 10$$-$$3 mole of H2(g) is used to compress 1.00 mole of a monoatomic ideal gas in a thermally insulated container. What is the change in the temperature (in K) of the ideal gas?
The standard reduction potentials for the two half-cells are given below :
$${O_2}(g) + 4{H^ + }(aq) + 4{e^ - }\buildrel {} \over \longrightarrow 2{H_2}O(l),$$
$${E^o} = 1.23V$$
$$2{H^ + }(aq) + 2{e^ - }\buildrel {} \over \longrightarrow {H_2}(g),$$
$${E^o} = 0.00\,V$$
Use, $$F = 96500\,C\,mo{l^{ - 1}}$$, $$R = 8.314\,J\,mo{l^{ - 1}}\,{K^{ - 1}}$$.
$${H_2}(g) + {1 \over 2}{O_2}(g)\buildrel {} \over \longrightarrow {H_2}O(l)$$
The work derived from the cell on the consumption of 1.0 $$ \times $$ 10$$-$$3 mole of H2(g) is used to compress 1.00 mole of a monoatomic ideal gas in a thermally insulated container. What is the change in the temperature (in K) of the ideal gas?
The standard reduction potentials for the two half-cells are given below :
$${O_2}(g) + 4{H^ + }(aq) + 4{e^ - }\buildrel {} \over \longrightarrow 2{H_2}O(l),$$
$${E^o} = 1.23V$$
$$2{H^ + }(aq) + 2{e^ - }\buildrel {} \over \longrightarrow {H_2}(g),$$
$${E^o} = 0.00\,V$$
Use, $$F = 96500\,C\,mo{l^{ - 1}}$$, $$R = 8.314\,J\,mo{l^{ - 1}}\,{K^{ - 1}}$$.
Answer
13.32
16
Aluminium reacts with sulphuric acid to form aluminium sulphate and hydrogen. What is the volume of hydrogen gas in litre (L) produced at 300 K and 1.0 atm pressure, when 5.4 g of aluminium and 50.0 mL of 5.0 M sulphuric acid are combined for the reaction?
(Use molar mass of aluminium as 27.0 g mol$$-$$1, R = 0.082 atm L mol$$-$$1 K$$-$$1)
(Use molar mass of aluminium as 27.0 g mol$$-$$1, R = 0.082 atm L mol$$-$$1 K$$-$$1)
Answer
6.15
17
$$_{92}^{238}U$$ is known to undergo radioactive decay to form $$_{82}^{206}Pb$$ by emitting alpha and beta particles. A rock initially contained 68 $$ \times $$ 10$$-$$6 g of $$_{92}^{238}U$$. If the number of alpha particles that it would emit during its radioactive decay of $$_{92}^{238}U$$ to $$_{82}^{206}Pb$$ in three half-lives is Z $$ \times $$ 1018, then what is the value of Z?
Answer
1.2