JEE Advance - Chemistry (2020 - Paper 1 Offline - No. 1)
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
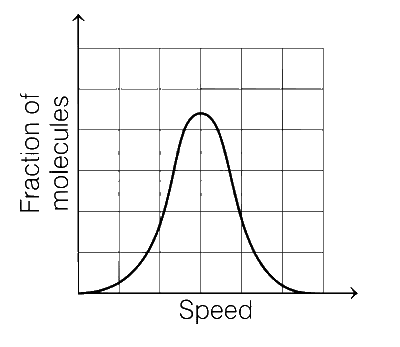

1 : 1 : 1
1 : 1 : 1.224
1 : 1.128 : 1.224
1 : 1.128 : 1
Explanation
Fraction of molecules vs velocity graph is Maxwell distribution curve.
This curve is slightly unsymmetrical as shown below.
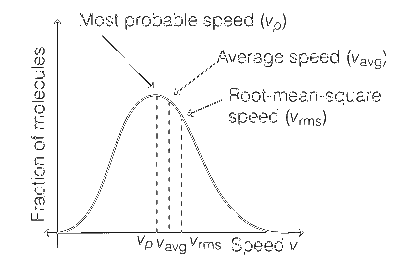
This curve is slightly unsymmetrical as shown below.

The ratio of most probable, the average and the root mean square speeds for this graph is 1 : 1.128 : 1.224
But the graph in question is completely symmetrical. Therefore, the most probable and the average speed will be same here but root mean square speed will be greater than average speed. So, the correct ratio is 1 : 1 : 1.224.
Comments (0)


