JEE Advance - Chemistry (2020 - Paper 1 Offline - No. 8)
With respect to the compounds I-V, choose the correct statement(s).
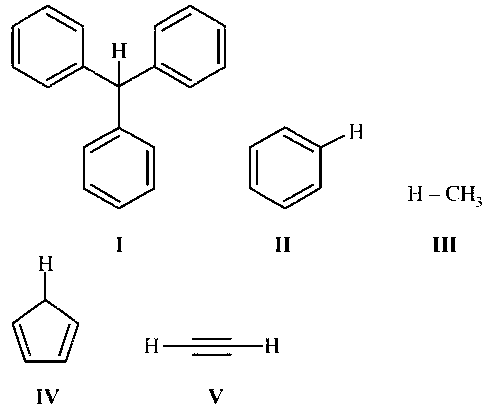

The acidity of compound I is due to delocalisation in the conjugate base.
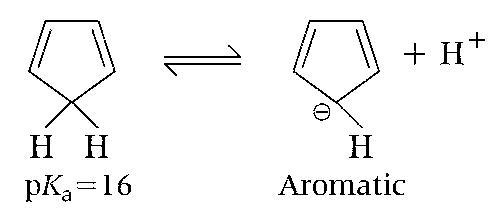
The conjugate base of compound IV is aromatic.
Compound II becomes more acidic, when it has a $$-$$NO2 substituent.
The acidity of compounds follows the order
I > IV > V > II > III.
I > IV > V > II > III.
Explanation
Triphenylmethane (I) is acidic because its conjugate base is stabilised by resonance.
$${(Ph)_3}CH\buildrel {} \over \longrightarrow \mathop {{{(Ph)}_3}C}\limits_{Triphenylmethyl\,carbanion} + {H^ + }$$
Cyclopentadiene (IV) is acidic because its conjugate base is aromatic.
Nitrobenzene is more acidic than benzene because nitro group is electron withdrawing. It will stabilise the conjugate base of benzene by $$-$$R and $$-$$I effect.
The acidic strength order on the basis of pKa data is
IV > V > I > II > III.
Hence, the correct options are (a), (b) and (c) only.
$${(Ph)_3}CH\buildrel {} \over \longrightarrow \mathop {{{(Ph)}_3}C}\limits_{Triphenylmethyl\,carbanion} + {H^ + }$$
Cyclopentadiene (IV) is acidic because its conjugate base is aromatic.

Nitrobenzene is more acidic than benzene because nitro group is electron withdrawing. It will stabilise the conjugate base of benzene by $$-$$R and $$-$$I effect.
The acidic strength order on the basis of pKa data is
IV > V > I > II > III.
Hence, the correct options are (a), (b) and (c) only.
Comments (0)


