JEE Advance - Chemistry (2020 - Paper 1 Offline - No. 18)
In the following reaction, compound Q is obtained from compound P via an ionic intermediate

What is the degree of unsaturation of Q?

What is the degree of unsaturation of Q?
Answer
18
Explanation
Compound (P) on treatment with concentrated H2SO4, gives intermediate compound, on delocalisation, gives a coloured compound Q.
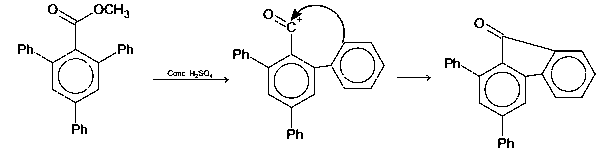
Number of rings = 4 + 1 = 5
$$\pi $$-bonds = 4 $$ \times $$ 3 + 1 = 13
Thus, the degree of unsaturation of Q is 18.

Number of rings = 4 + 1 = 5
$$\pi $$-bonds = 4 $$ \times $$ 3 + 1 = 13
Thus, the degree of unsaturation of Q is 18.
Comments (0)


