JEE Advance - Chemistry (2020 - Paper 1 Offline - No. 3)
A colourless aqueous solution contains nitrates of two metals, X and Y. When it was added to an aqueous solution of NaCl, a white precipitate was formed. This precipitate was found to be partly soluble in hot water to give a residue P and a solution Q. The residue P was soluble in aqueous NH3 and also in excess sodium thiosulphate. The hot solution Q gave a yellow precipitate with KI. The metals X and Y, respectively, are
Ag and Pb
Ag and Cd
Cd and Pb
Cd and Zn
Explanation
X : Ag Y : Pb P : AgCl Q : PbCl2
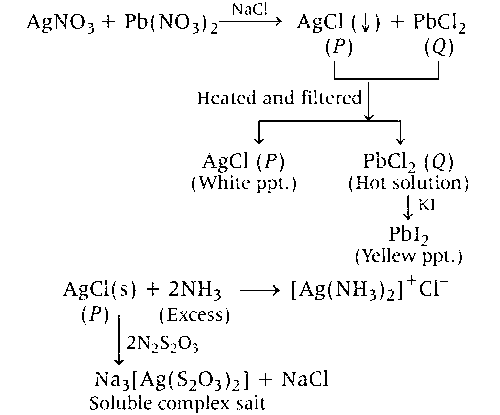
Hence, the correct option is (a).

Hence, the correct option is (a).
Comments (0)


