JEE Advance - Chemistry (2020 - Paper 1 Offline - No. 11)
With respect to hypochlorite, chlorate and perchlorate ions, choose the correct statement(s).
The hypochlorite ion is the strongest conjugate base.
The molecular shape of only chlorate ion is influenced by the lone pair of electrons of Cl.
The hypochlorite and chlorate ions disproportionate to give rise to identical set of ions.
The hypochlorite ion oxidises the sulphite ion.
Explanation
(a) Order of acid strength different oxyacids of chlorine are :
$$\mathop {HClO}\limits_{(Hypochlorous\,acid)}^{( + 1)} < \mathop {HCl{O_3}}\limits_{(Chloric\,acid)}^{( + 5)} < \mathop {HCl{O_4}}\limits_{(Perchloric\,acid)}^{( + 7)} $$
Weak acid have strong conjugate base thus hypochlorite ion has strongest conjugate base. Therefore, statement (a) is correct.
(b) Hypochlorite ion is linear and perchlorate ion is tetrahedral and there is no effect of lone pair on hypochlorite ion. Thus, statement (b) is correct.
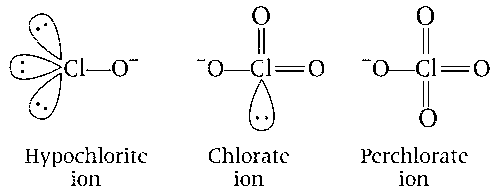
(c) In the disproportionation reaction, chlorate ion Cl(+5) is oxidised to perchlorate, Cl(+7) and reduce to chloride, Cl($$-$$1).
$$4ClO_3^ - \mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} 3ClO_4^ - + C{l^ - }$$
While in hypochlorite ion, chlorite ion Cl(+1) is oxidised to chlorate, Cl(+5) and reduced to chloride, Cl($$-$$1) ion.
$$3ClO_{}^ - \mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} ClO_3^ - + 2C{l^ - }$$
Thus, statement (c) is incorrect.
(d) The hypochlorite ion oxidises the sulphite ion to sulphate ion, because HOCl is the strongest oxidising Cloxyacids,
$$Cl{O^ - } + SO_3^{2 - }\buildrel {} \over \longrightarrow SO_4^{2 - } + C{l^ - }$$
Thus, statement (d) is correct.
$$\mathop {HClO}\limits_{(Hypochlorous\,acid)}^{( + 1)} < \mathop {HCl{O_3}}\limits_{(Chloric\,acid)}^{( + 5)} < \mathop {HCl{O_4}}\limits_{(Perchloric\,acid)}^{( + 7)} $$
Weak acid have strong conjugate base thus hypochlorite ion has strongest conjugate base. Therefore, statement (a) is correct.
(b) Hypochlorite ion is linear and perchlorate ion is tetrahedral and there is no effect of lone pair on hypochlorite ion. Thus, statement (b) is correct.

(c) In the disproportionation reaction, chlorate ion Cl(+5) is oxidised to perchlorate, Cl(+7) and reduce to chloride, Cl($$-$$1).
$$4ClO_3^ - \mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} 3ClO_4^ - + C{l^ - }$$
While in hypochlorite ion, chlorite ion Cl(+1) is oxidised to chlorate, Cl(+5) and reduced to chloride, Cl($$-$$1) ion.
$$3ClO_{}^ - \mathrel{\mathop{\kern0pt\longrightarrow} \limits_{}} ClO_3^ - + 2C{l^ - }$$
Thus, statement (c) is incorrect.
(d) The hypochlorite ion oxidises the sulphite ion to sulphate ion, because HOCl is the strongest oxidising Cloxyacids,
$$Cl{O^ - } + SO_3^{2 - }\buildrel {} \over \longrightarrow SO_4^{2 - } + C{l^ - }$$
Thus, statement (d) is correct.
Comments (0)


