JEE Advance - Chemistry (2020 - Paper 1 Offline - No. 10)
Choose the correct statement(s) among the following :
[FeCl4]$$-$$ has tetrahedral geometry.
[Co(en)(NH3)2Cl2]+ has 2 geometrical isomers.
[FeCl4]$$-$$ has higher spin-only magnetic moment than [Co(en)(NH3)2Cl2]+.
The cobalt ion in [Co(en)(NH3)2Cl2]+ has sp3d2 hybridisation.
Explanation
(a) In [FeCl4]-, oxidation number of Fe atom = +3
Electronic configuration of Fe in ground state = 3d64s2
Electronic configuration of
Fe3+ = 3d54s04p0
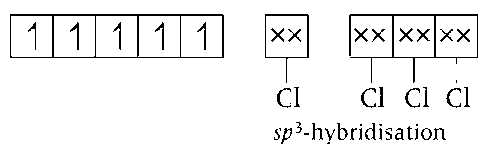
Thus, [FeCl4]- has tetrahedral geometry.
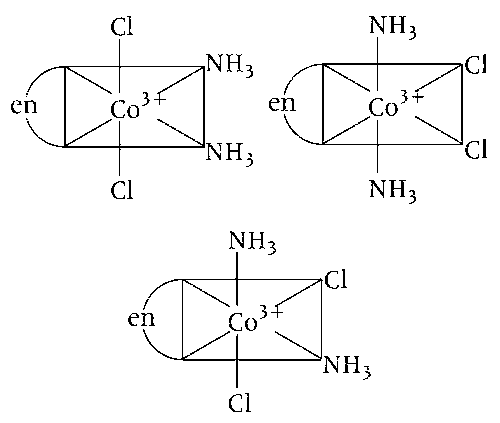
(b) [Co(en)(NH3)2Cl2]+ have three geometrical isomers. Thus, statement (b) is incorrect.
(c) Fe3+ in [FeCl4]- is sp3-hybridised with 5 unpaired electrons. (higher spin-only magnetic moment
= $$\sqrt {n(n + 2)} $$ = 5.92 BM). While Co3+ in [Co(en)(NH3)2Cl2]+ is d2sp3-hybridised with zero unpaired electrons (low spin-only magnetic moment = $$\sqrt {n(n + 2)} $$ = 0 BM).
Thus, the statement (c) is correct.
(d) Co3+[Co(en)(NH3)2Cl2]+
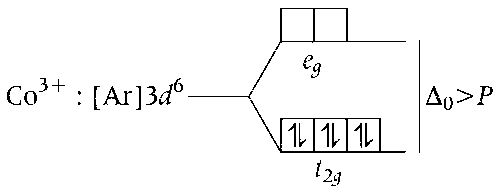
Co3+ in [Co(en)(NH3)2Cl2]+ is d2sp3-hybridised and has octahedral geometry with 0 unpaired electron. Thus, statement (d) is incorrect.
Electronic configuration of Fe in ground state = 3d64s2
Electronic configuration of
Fe3+ = 3d54s04p0

Thus, [FeCl4]- has tetrahedral geometry.
(b) [Co(en)(NH3)2Cl2]+ have three geometrical isomers. Thus, statement (b) is incorrect.

(c) Fe3+ in [FeCl4]- is sp3-hybridised with 5 unpaired electrons. (higher spin-only magnetic moment
= $$\sqrt {n(n + 2)} $$ = 5.92 BM). While Co3+ in [Co(en)(NH3)2Cl2]+ is d2sp3-hybridised with zero unpaired electrons (low spin-only magnetic moment = $$\sqrt {n(n + 2)} $$ = 0 BM).
Thus, the statement (c) is correct.
(d) Co3+[Co(en)(NH3)2Cl2]+

Co3+ in [Co(en)(NH3)2Cl2]+ is d2sp3-hybridised and has octahedral geometry with 0 unpaired electron. Thus, statement (d) is incorrect.
Comments (0)


