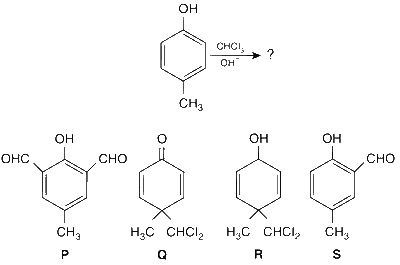
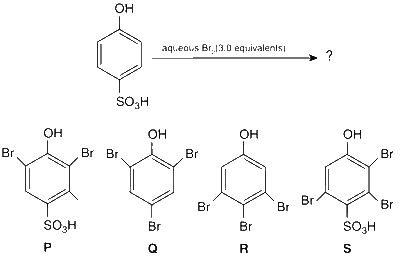
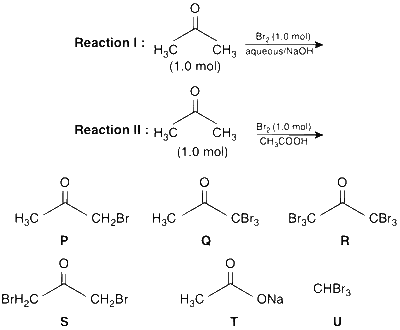
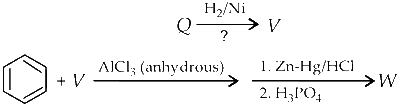
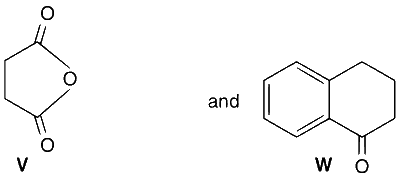
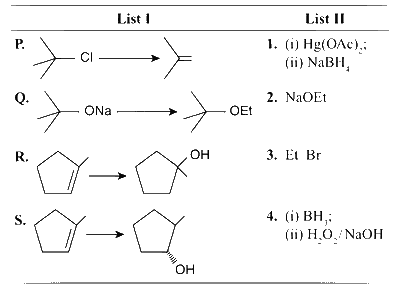
JEE Advance - Chemistry (2013 - Paper 2 Offline)
3
The standard reduction potential data at 25oC is given below:
Eo (Fe3+ , Fe2+) = +0.77V;
Eo (Fe2+ , Fe) = -0.44V;
Eo (Cu2+ , Cu) = +0.34V;
Eo (Cu+ , Cu) = +0.52V;
Eo [O2(g) + 4H+ + 4e- $$\to$$ 2H2O] = +1.23V;
Eo [O2(g) + 2H2O + 4e- $$\to$$ 4OH-] = +0.40 V
Eo (Cr3+ , Cr) = -0.74V;
Eo (Cr2+ , Cr) = -0.91V;
Match Eo of the redox pair in List – I with the values given in List – II and select the correct answer using the code given below the lists:
List - I
P. Eo (Fe3+ , Fe)
Q. Eo (4H2O $$\leftrightharpoons$$ 4H+ + 4OH-)
R. Eo (Cu2+ + Cu $$\to$$ 2Cu+)
S. Eo (Cr3+, Cr2+)
List - II
1. -0.18 V
2. -0.4 V
3. -0.04 V
4. -0.83 V
Eo (Fe3+ , Fe2+) = +0.77V;
Eo (Fe2+ , Fe) = -0.44V;
Eo (Cu2+ , Cu) = +0.34V;
Eo (Cu+ , Cu) = +0.52V;
Eo [O2(g) + 4H+ + 4e- $$\to$$ 2H2O] = +1.23V;
Eo [O2(g) + 2H2O + 4e- $$\to$$ 4OH-] = +0.40 V
Eo (Cr3+ , Cr) = -0.74V;
Eo (Cr2+ , Cr) = -0.91V;
Match Eo of the redox pair in List – I with the values given in List – II and select the correct answer using the code given below the lists:
List - I
P. Eo (Fe3+ , Fe)
Q. Eo (4H2O $$\leftrightharpoons$$ 4H+ + 4OH-)
R. Eo (Cu2+ + Cu $$\to$$ 2Cu+)
S. Eo (Cr3+, Cr2+)
List - II
1. -0.18 V
2. -0.4 V
3. -0.04 V
4. -0.83 V
Answer
(D)
P - 3; Q - 4; R - 1; S - 2
4
An aqueous solution of X is added slowly to an aqueous solution of Y as shown in List – I. The variation in
conductivity of these reactions in List – II. Match List – I with List – II and select the correct answer using
the code given below the lists:
List - I
P. $$\mathop {(C{}_2{H_5}){}_3N}\limits_X $$ + $$\mathop {C{H_3}COOH}\limits_Y $$
Q. $$\mathop {KI(0.1M)}\limits_X $$ + $$\mathop {AgN{O_3}(0.01M)}\limits_Y $$
R. $$\mathop {C{H_3}COOH}\limits_X $$ + $$\mathop {KOH}\limits_Y $$
S. $$\mathop {NaOH}\limits_X $$ + $$\mathop {HI}\limits_Y $$
List - II
1. Conductivity decreases then increases
2. Conductivity decreases then does not change much
3. Conductivity increases then does not change much
4. Conductivity does not change much then increases
List - I
P. $$\mathop {(C{}_2{H_5}){}_3N}\limits_X $$ + $$\mathop {C{H_3}COOH}\limits_Y $$
Q. $$\mathop {KI(0.1M)}\limits_X $$ + $$\mathop {AgN{O_3}(0.01M)}\limits_Y $$
R. $$\mathop {C{H_3}COOH}\limits_X $$ + $$\mathop {KOH}\limits_Y $$
S. $$\mathop {NaOH}\limits_X $$ + $$\mathop {HI}\limits_Y $$
List - II
1. Conductivity decreases then increases
2. Conductivity decreases then does not change much
3. Conductivity increases then does not change much
4. Conductivity does not change much then increases
Answer
(A)
P - 3; Q - 4; R - 2; S - 1
19
The unbalanced chemical reactions given in List I show missing reagent or condition (?) which are provided in List II. Match List I with List II and select the correct answer using the code given below the lists :
| List I | List II | ||
|---|---|---|---|
| P. | $$Pb{O_2} + {H_2}S{O_4}\buildrel ? \over \longrightarrow PbS{O_4} + {O_2} + Other\,products$$ |
1. | NO |
| Q. | $$N{a_2}{S_2}{O_3} + {H_2}O\buildrel ? \over \longrightarrow NaHS{O_4} + Other\,products$$ |
2. | $${I_2}$$ |
| R. | $${N_2}{H_4}\buildrel ? \over \longrightarrow {N_2} + Other\,products$$ |
3. | Warm |
| S. | $$Xe{F_2}\buildrel ? \over \longrightarrow Xe + Other\,products$$ |
4. | $$C{l_2}$$ |
Answer
(D)
P-3, Q-4, R-2, S-1