JEE Advance - Chemistry (2013 - Paper 2 Offline - No. 5)
CaCO3(s) $$\leftrightharpoons$$ CaO(s) + CO2(g).
For this equilibrium, the correct statement(s) is (are)
Explanation
The thermal decomposition of limestone (CaCO3) is represented as:
$$ \mathrm{CaCO}_3(\mathrm{s}) \rightleftharpoons \mathrm{CaO}(\mathrm{s}) + \mathrm{CO}_2(\mathrm{g}), \Delta_r \mathrm{H} = \text {positive} $$
The reaction is endothermic, meaning it requires heat to proceed.
(i) The forward reaction is endothermic (ΔH > 0), indicating heat absorption, while the reverse reaction is exothermic (ΔH < 0), indicating heat release.
Therefore, increasing the temperature will favor the forward reaction, leading to more CaCO3 dissociation. Conversely, decreasing the temperature will favor the reverse reaction. Option (A) is correct.
The equilibrium constant (K) varies with temperature but does not depend on the concentration of any species in an aqueous solution or the pressure of gases such as CO2. Option (B) is correct.
(ii) The equilibrium constant (K) for the reaction is expressed as:
$$ \mathrm{K}_{\mathrm{C}} = [\mathrm{CO}_2] $$
Because CaCO3 and CaO are solids, their concentrations do not affect the equilibrium constant. Option (B) is correct.
(iii) At a given temperature, the equilibrium constant in terms of pressure (KP) depends on the vapour pressure of CO2.
$$ \mathrm{K}_{\mathrm{P}} = \mathrm{P}_{\mathrm{CO}_2} $$
The equilibrium constant (KC) also depends on the pressure of CO2:
$$ \mathrm{K}_{\mathrm{C}} = \mathrm{P}_{\mathrm{CO}_2} (\mathrm{RT})^{-\Delta n} $$
Option (C) is correct.
The temperature dependence of the Arrhenius constant is given by:
$$ \ln \frac{\mathrm{K}_2}{\mathrm{K}_1} = \frac{\Delta_r \mathrm{H}}{\mathrm{R}} \left[ \frac{1}{\mathrm{T}_2} - \frac{1}{\mathrm{T}_1} \right] $$
(iv) A catalyst alters the activation energy of the reaction, thus affecting its speed. However, it does not change the enthalpy or heat of the reactants and products. As a result, the enthalpy of the reaction ($\Delta_r H$) remains unchanged. Option (D) is correct.
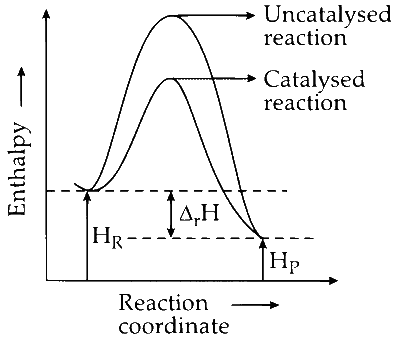
ΔrH is the enthalpy of the reaction.
Comments (0)


