JEE Advance - Chemistry (2013 - Paper 2 Offline - No. 4)
List - I
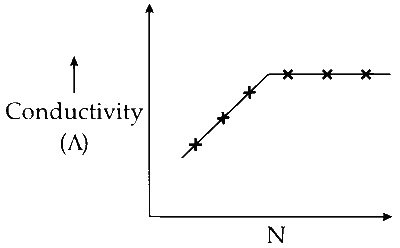
P. $$\mathop {(C{}_2{H_5}){}_3N}\limits_X $$ + $$\mathop {C{H_3}COOH}\limits_Y $$
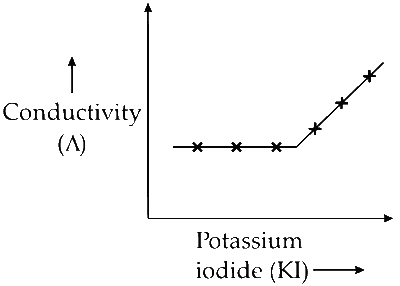
Q. $$\mathop {KI(0.1M)}\limits_X $$ + $$\mathop {AgN{O_3}(0.01M)}\limits_Y $$
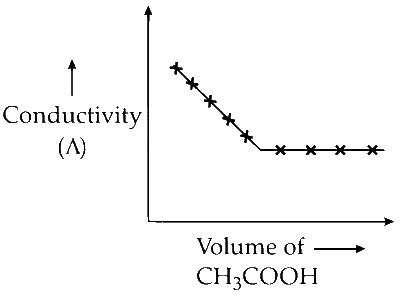
R. $$\mathop {C{H_3}COOH}\limits_X $$ + $$\mathop {KOH}\limits_Y $$
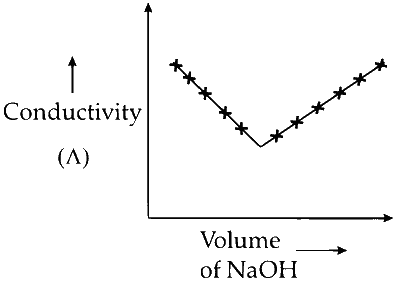
S. $$\mathop {NaOH}\limits_X $$ + $$\mathop {HI}\limits_Y $$
List - II
1. Conductivity decreases then increases
2. Conductivity decreases then does not change much
3. Conductivity increases then does not change much
4. Conductivity does not change much then increases
Explanation
```html
(P) The weak acid (Y) is partially dissociated as follows:
$$ \mathrm{CH}_3 \mathrm{COOH}(aq) \rightleftharpoons \mathrm{CH}_3 \mathrm{COO}^{-}(aq) + \mathrm{H}^{+}(aq) $$
(a) Adding the base triethylamine will result in its protonation by $\mathrm{H}^{+}$. As more $\mathrm{H}^{+}$ ions are consumed, the reaction shifts forward, producing $\mathrm{CH}_3 \mathrm{COO}^{-}$ ions and protonated amine, increasing the solution's conductivity.
$$ (\mathrm{C}_2 \mathrm{H}_5)_3 \ddot{\mathrm{N}} + \mathrm{H}^{+} \rightarrow (\mathrm{C}_2 \mathrm{H}_5)_3 \mathrm{NH}^{+} $$
Net equation:
$$ \mathrm{CH}_3 \mathrm{COOH}(aq) + (\mathrm{C}_2 \mathrm{H}_5)_3 \ddot{\mathrm{N}} \rightarrow (\mathrm{C}_2 \mathrm{H}_5)_3 \stackrel{+}{\mathrm{N}}\mathrm{H} + \mathrm{CH}_3 \mathrm{COO}^{-} $$
(b) Once all the acid is neutralized, adding more $(\mathrm{C}_2 \mathrm{H}_5)_3 \mathrm{N}$ will not further affect the solution's conductivity.
Hence, initially conductivity increases and then remains constant.
Option (P) in List-I matches with option 3 in List-II.
(Q) The reaction between potassium iodide (KI) and silver nitrate $(\mathrm{AgNO}_3)$ occurs as follows:
$$ \mathrm{KI}(0.1 \, \mathrm{M}) + \mathrm{AgNO}_3(0.01 \, \mathrm{M}) \rightarrow \mathrm{KNO}_3 + \mathrm{AgI}_{(\mathrm{ppt})} $$
(a) As KI is added to $\mathrm{AgNO}_3$, silver iodide precipitates, and conductivity due to $\mathrm{AgNO}_3$ decreases, but potassium nitrate formation increases the solution's conductivity. These effects balance out, so conductivity does not change much initially.
(b) After all $\mathrm{AgNO}_3$ is consumed, adding more KI, a strong electrolyte, will increase the solution's conductivity.
Hence, initially conductivity does not change much and then increases.
(R) The weak acid $\mathrm{CH}_3 \mathrm{COOH}$ dissociates as follows:
$$ \mathrm{CH}_3 \mathrm{COOH}(aq) \rightleftharpoons \mathrm{CH}_3 \mathrm{COO}^{-}(aq) + \mathrm{H}^{+}(aq) $$
When it is added to potassium hydroxide (KOH), a strong electrolyte, the following reaction takes place:
$$ \mathrm{CH}_3 \mathrm{COOH} + \mathrm{KOH} \rightarrow \mathrm{CH}_3 \mathrm{COO}^{-} \mathrm{K}^{+} + \mathrm{H}_2 \mathrm{O} $$
The potassium ion (K$^+$) from KOH, which has a higher migration velocity, is replaced by the hydrogen and acetate ion $\mathrm{CH}_3 \mathrm{COO}^{-}$, which has lower migration velocity. Consequently, conductivity decreases. After all KOH is neutralized by the weak acetic acid $\mathrm{CH}_3 \mathrm{COOH}$, adding more acid will increase conductivity only slightly due to its lower degree of dissociation. Hence, initially the conductivity decreases but then changes very little.
(S) Hydrogen iodide (HI) is a strong acid and completely dissociates into hydrogen and iodide ions. Addition of sodium hydroxide (NaOH) forms sodium iodide and water. The hydrogen ions, which have high migration velocity, are replaced by slower-moving sodium ions, causing a decrease in conductivity. After all the HI is neutralized, conductivity increases with further addition of NaOH due to the increased concentration of $\mathrm{OH}^{-}$ ions.
P - 3; Q - 4; R - 2; S - 1
```
Comments (0)


