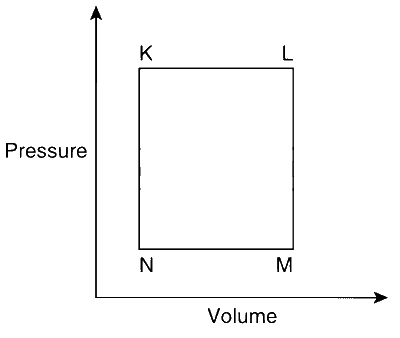
JEE Advance - Chemistry (2013 - Paper 2 Offline - No. 15)
The succeeding operations that enable this transformation of states are
Heating, cooling, heating, cooling.
Cooling, heating, cooling, heating.
Heating, cooling, cooling, heating.
Cooling, heating, heating, cooling.
Explanation
From the graph, as the term pV increases, the temperature increases; and when pV decreases the temperature decreases.
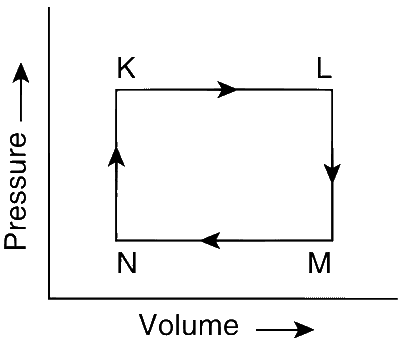
The term PV increases K$$\to$$L, so this involves heating and is an isobaric process.
It increases from N$$\to$$K, so this involves heating and is an isochoric process.
It decreases from M$$\to$$N, so this involves cooling and is an isobaric process.
It decreases from L$$\to$$M, so this involves cooling and is an isochoric process.
Comments (0)