JEE Advance - Chemistry (2013 - Paper 2 Offline - No. 10)
The correct statement(s) about O3 is(are)
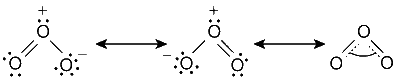
O-O bond lengths are equal.
Thermal decomposition of O3 is endothermic.
O3 is diamagnetic in nature.
O3 has a bent structure.
Explanation
As all electrons are paired, ozone is diamagnetic in nature. The structure is bent or V-shaped. The structure of ozone is resonance hybrid of the two structures with a delocalised p-orbital which covers all three atoms. Because of this, the two O-O bond lengths are equal.
Comments (0)


