JEE Advance - Chemistry (2013 - Paper 2 Offline - No. 13)
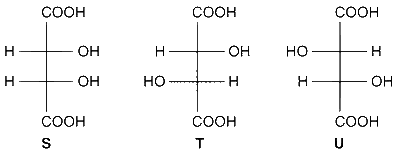
Compounds formed from P and Q are, respectively,
Optically active S and optically active pair (T, U).
Optically inactive S and optically inactive pair (T, U).
Optically active pair (T, U) and optically active S.
Optically inactive pair (T, U) and optically inactive S.
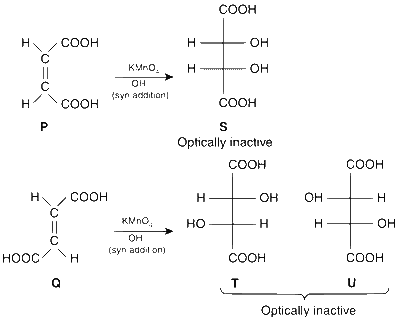
Explanation
The reactions involved are
Comments (0)