JEE Advance - Chemistry (2013 - Paper 2 Offline - No. 16)
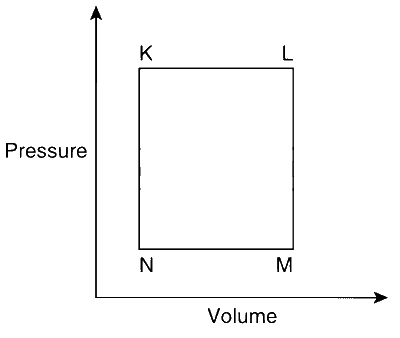
The pair of isochoric processes among the transformation of states is
K to L and L to M
L to M and N to K
L to M and M to N
M to N and N to K
Explanation
From the above solution, we have
From K$$\to$$L : heating (isobaric)
From L$$\to$$M : cooling (isochoric)
From M$$\to$$N : cooling (isobaric)
From N$$\to$$K : heating (isochoric)
Comments (0)