JEE Advance - Chemistry (2009 - Paper 2 Offline)
1
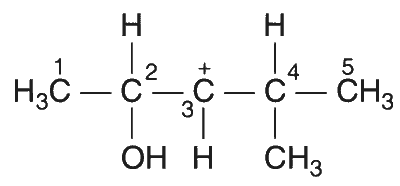
For a first-order reaction A $$\to$$ P, the temperature (T) dependent rate constant (k) was found to follow the equation $$\log k = - (2000){1 \over T} + 6.0$$. The pre-exponential factor A and activation energy $$E_a$$, respectively, are
Answer
(D)
$$1.0\times10^6~\mathrm{s^{-1}}$$ and 38.3 kJ mol$$^{-1}$$
5
For the reduction of NO$$_3^ - $$ ion in an aqueous solution, E$$^0$$ is + 0.96 V. Values of E$$^0$$ for some metal ions are given below:
$$\matrix{ {{V^{2 + }}(aq.) + 2{e^ - } \to V} & {{E^0} = - 1.19\,V} \cr {F{e^{3 + }}(aq.) + 3{e^ - } \to Fe} & {{E^0} = - 0.04\,V} \cr {A{u^{3 + }}(aq) + 3{e^ - } \to Au} & {{E^0} = + 1.40\,V} \cr {H{g^{2 + }}(aq) + 2{e^ - } \to Hg} & {{E^0} = + 0.86\,V} \cr } $$
The pair(s) of metals that is (are) oxidized by NO$$_3^ - $$ in aqueous solution is(are)
Answer
A
B
D
10
Match each of the reactions given in Column I with the corresponding product(s) given in Column II:
| Column I | Column II | ||
|---|---|---|---|
| (A) | $$\mathrm{Cu+dil.~HNO_3}$$ | (P) | $$\mathrm{NO}$$ |
| (B) | $$\mathrm{Cu+conc.~HNO_3}$$ | (Q) | $$\mathrm{NO_2}$$ |
| (C) | $$\mathrm{Zn+dil.~HNO_3}$$ | (R) | $$\mathrm{N_2O}$$ |
| (D) | $$\mathrm{Zn+conc.~HNO_3}$$ | (S) | $$\mathrm{Cu(NO_3)_2}$$ |
| (T) | $$\mathrm{Zn(NO_3)_2}$$ |
Answer
(C)
(A)$$\to$$(P), (S); (B)$$\to$$(Q), (S); (C)$$\to$$(R), (T); (D)$$\to$$(Q), (T)
11
Match each of the compounds given in Column I with the reaction(s), that they can undergo, given in Column II.
| Column I | Column II | ||
|---|---|---|---|
| (A) | 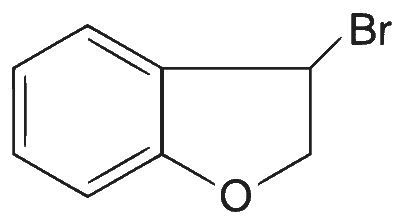 |
(P) | Nucleophilic substitution |
| (B) | 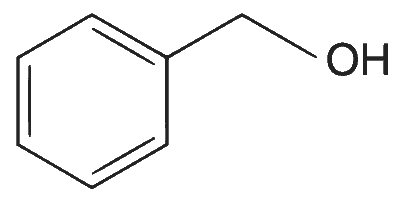 |
(Q) | Elimination |
| (C) | 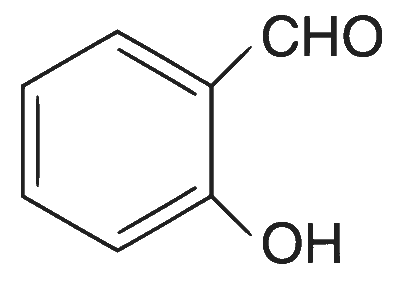 |
(R) | Nucleophilic addition |
| (D) | 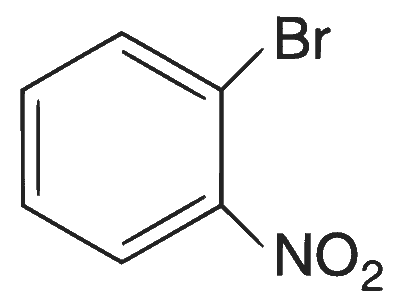 |
(S) | Esterification with acetic anhydride |
| (T) | Dehydrogenation |
Answer
(D)
(A)$$\to$$(P), (Q), (T); (B)$$\to$$(P), (S), (T); (C)$$\to$$(R), (S); (D)$$\to$$(P)
12
In a constant volume calorimeter, 3.5 g of a gas with molecular weight 28 was burnt in excess oxygen at 298.0 K. The temperature of the calorimeter was found to increase from 298.0 K to 298.45 K due to the combustion process. Given that the heat capacity of the calorimeter is 2.5 kJ K$$^{-1}$$, the numerical value for the enthalpy of combustion of the gas in kJ mol$$^{-1}$$ is ____________.
Answer
9