JEE Advance - Chemistry (2009 - Paper 2 Offline - No. 14)
The dissociation constant of a substituted benzoic acid at 25$$^\circ$$C is 1.0 $$\times$$ 10$$^{-4}$$. The pH of a 0.01 M solution of its sodium salt is __________.
Answer
8
Explanation
Given that
$${K_a}({C_6}{H_5}COOH) = 1 \times {10^{ - 4}}$$.
pH of 0.01 M $${C_6}{H_5}COONa$$.
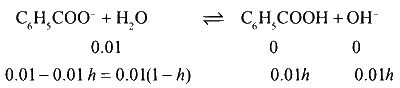
$${K_h} = {{{K_w}} \over {{K_a}}} = {{0.01\,{h^2}} \over {1 - h}}$$
$$ \Rightarrow {{{{10}^{ - 14}}} \over {{{10}^{ - 4}}}} = {{{{10}^{ - 2}}{h^2}} \over {1 - h}}$$
$$1 - h$$ is approximately equal to 1.
[OH$$^-$$] = $$0.01h$$ = 0.01 $$\times$$ 10$$^{-4}$$ = 10$$^{-6}$$
[H$$^+$$] = 10$$^{-8}$$
pH = 8
Comments (0)


