JEE Advance - Chemistry (2009 - Paper 2 Offline - No. 8)
The nitrogen oxide(s) that contain(s) N-N bond(s) is(are)
N$$_2$$O
N$$_2$$O$$_3$$
N$$_2$$O$$_4$$
N$$_2$$O$$_5$$
Explanation
The bond formation in the given oxides of nitrogen is as follows.
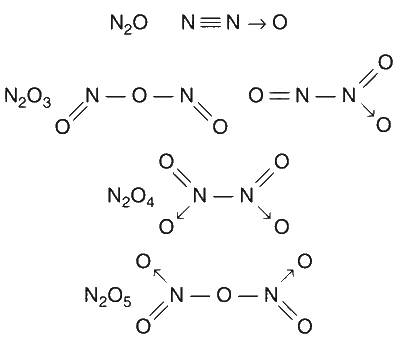
Thus N-N bond exists in N$$_2$$O, N$$_2$$O$$_3$$ and N$$_2$$O$$_4$$.
Comments (0)


