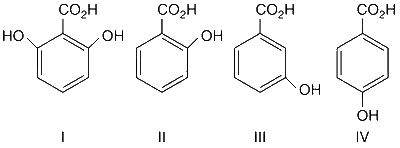
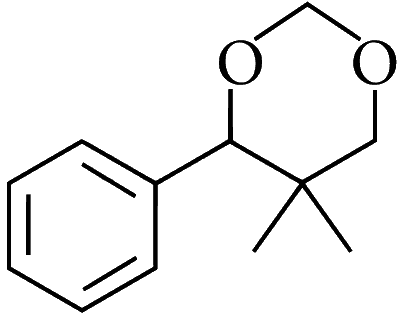
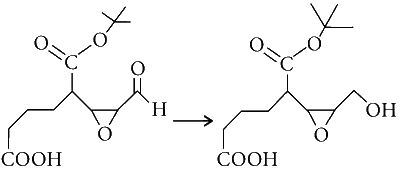
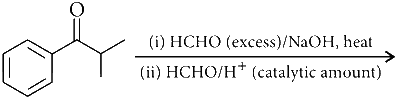JEE Advance - Chemistry (2016 - Paper 2 Offline)
2
For the following electrochemical cell at 298 K
Pt(s) | H2 (g, 1 bar) | H+ (aq, 1 M) || M4+ (aq), M2+ (aq) | Pt (s)
Ecell = 0.092 V when $${{\left[ {{M^{2 + }}(aq)} \right]} \over {\left[ {{M^{4 + }}(aq)} \right]}}$$ = 10x
Give, $$E_{{M^{4+}}/{M^{2 + }}}^o$$ = 0.151 V; 2.303 RT/F = 0.059 V
Pt(s) | H2 (g, 1 bar) | H+ (aq, 1 M) || M4+ (aq), M2+ (aq) | Pt (s)
Ecell = 0.092 V when $${{\left[ {{M^{2 + }}(aq)} \right]} \over {\left[ {{M^{4 + }}(aq)} \right]}}$$ = 10x
Give, $$E_{{M^{4+}}/{M^{2 + }}}^o$$ = 0.151 V; 2.303 RT/F = 0.059 V
The value of x is
Answer
(D)
2
5
Paragraph
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equations:
X2 (g) $$\leftrightharpoons$$ 2X (g)
The standard reaction Gibbs energy, $$\Delta _rG^o$$, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by $$\beta$$. Thus, $$\beta _{equilibrium}$$ is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally. (Given R = 0.083 L bar K-1 mol-1)
Question
The INCORRECT statement among the following for this reaction, is
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equations:
X2 (g) $$\leftrightharpoons$$ 2X (g)
The standard reaction Gibbs energy, $$\Delta _rG^o$$, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by $$\beta$$. Thus, $$\beta _{equilibrium}$$ is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally. (Given R = 0.083 L bar K-1 mol-1)
Question
The INCORRECT statement among the following for this reaction, is
Answer
(C)
$${{\beta _{equilibrium}}}$$ = 0.7
6
Paragraph
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equations:
X2 (g) $$\leftrightharpoons$$ 2X (g)
The standard reaction Gibbs energy, $$\Delta _rG^o$$, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by $$\beta$$. Thus, $$\beta _{equilibrium}$$ is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally. (Given R = 0.083 L bar K-1 mol-1)
Question
The equilibrium constant Kp for this reaction at 298 K, in terms of $$\beta _{equilibrium}$$, is
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equations:
X2 (g) $$\leftrightharpoons$$ 2X (g)
The standard reaction Gibbs energy, $$\Delta _rG^o$$, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by $$\beta$$. Thus, $$\beta _{equilibrium}$$ is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally. (Given R = 0.083 L bar K-1 mol-1)
Question
The equilibrium constant Kp for this reaction at 298 K, in terms of $$\beta _{equilibrium}$$, is
Answer
(B)
$${{8\beta _{equilibrium}^2} \over {4 - {\beta _{equilibrium}^2}}}$$
11
In the following reaction, sequence in aqueous solution, the species X, Y and Z, respectively, are
$${S_2}O_3^{2 - }\buildrel {A{g^ + }} \over \longrightarrow \mathop X\limits_{Clear\,solution} \buildrel {A{g^ + }} \over \longrightarrow \mathop Y\limits_{White\,precipitate} \buildrel {With\,time} \over \longrightarrow \mathop Z\limits_{Black\,precipitate} $$
Answer
(A)
[Ag(S2O3)2]3$$-$$, Ag2S2O3, Ag2S
12
The qualitative sketches I, II and III given below show the variation of surface tension with molar concentration of three different aqueous solutions of KCl, CH3OH and CH3(CH2)11 OSO$$_3^ - $$ Na+ at room temperature. The correct assignment of the sketches is
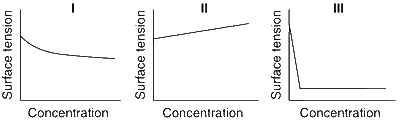
Answer
(D)
I. CH3OH
II. KCl
III. CH3(CH2)11 OSO$$_3^ - $$ Na+