JEE Advance - Chemistry (2016 - Paper 2 Offline - No. 16)
The nitrogen containing compound produced in the reaction of HNO3 with P4O10
can also be prepared by reaction of P4 and HNO3.
is diamagnetic.
contains one N$$-$$N bond.
reacts with Na metal producing a brown gas.
Explanation
The reaction of HNO3 and P4O10 produces N2O5.
4HNO3 + P4O10 $$\to$$ 2N2O5 + 4HPO3
The reaction of HNO3 with P4 does not yield N2O5.
P4 + 20 HNO3 $$\to$$ 4H3PO4 + 20 NO2 + 4 H2O
The structure of N2O5 has one N$$-$$O$$-$$N bond, but no N$$-$$N bond. It is diamagnetic in nature.
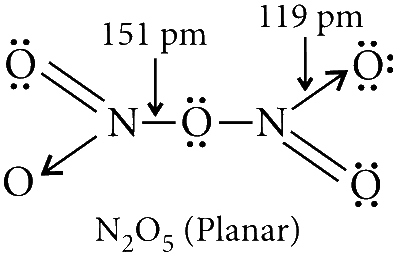
N2O5 reacts with sodium metal to produce NO2 (brown gas)
N2O5 + Na $$\to$$ NaNO3 + $$\mathop {N{O_2} \uparrow }\limits_{Brown\,gas} $$
Comments (0)


