JEE Advance - Chemistry (2016 - Paper 2 Offline - No. 9)
The correct order of acidity for the following compounds is
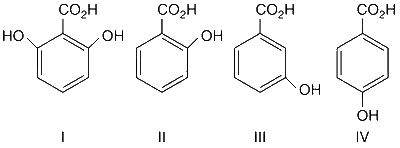
I > II > III > IV
III > I > II > IV
III > IV > II > I
I > III > IV > II
Explanation
Due to ortho-effect, (I) and (II) are stronger acid than (III) and (IV). Due to two ortho hydroxyl groups in (I), it is stronger acid than (II). (III) is a stronger acid than (IV) because at m-position, $$-$$OH group cannot exert its +R effect but can only exert its $$-$$I effect while at p-position, $$-$$OH group exerts its strong +R effect. Thus, the correct order of acidity is :
(I) > (II) > (III) > (IV)
Comments (0)


