JEE Advance - Chemistry (2016 - Paper 2 Offline - No. 12)
The qualitative sketches I, II and III given below show the variation of surface tension with molar concentration of three different aqueous solutions of KCl, CH3OH and CH3(CH2)11 OSO$$_3^ - $$ Na+ at room temperature. The correct assignment of the sketches is
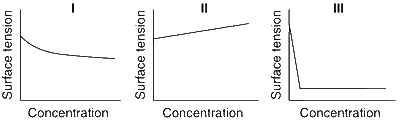
I : KCl
II : CH3OH
III : CH3(CH2)11 OSO$$_3^ - $$ Na+
I. CH3(CH2)11 OSO$$_3^ - $$ Na+
II. CH3OH
III. KCl
I. KCl
II. CH3(CH2)11 OSO$$_3^ - $$ Na+
III. CH3OH
I. CH3OH
II. KCl
III. CH3(CH2)11 OSO$$_3^ - $$ Na+
Explanation
I. (CH3OH) : Surface tension decreases as concentration increases.
II. (KCl) : Surface tension increases with concentration for ionic salt.
III. [CH3(CH2)11 OSO$$_3^ - $$ Na+] : It is an anionic detergent.
There is decrease in surface tension before micelle formation, and after CMC (Critical Micelle Concentration) is attained, no change in surface tension.
Comments (0)


