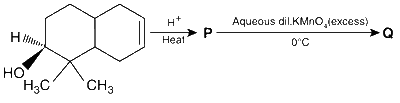
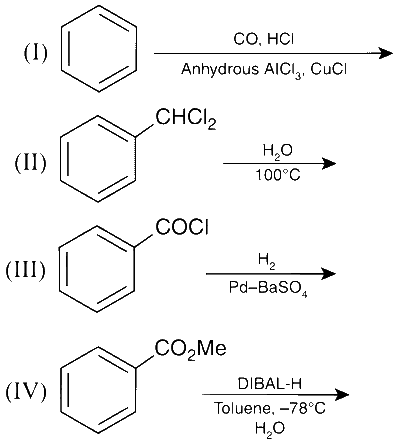
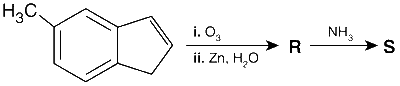
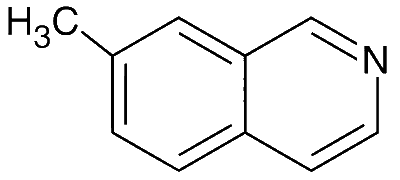
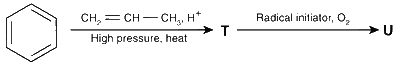
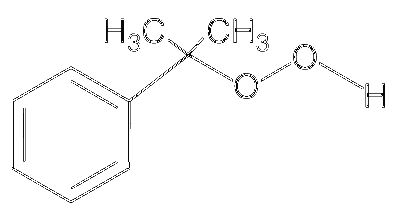
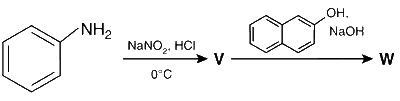
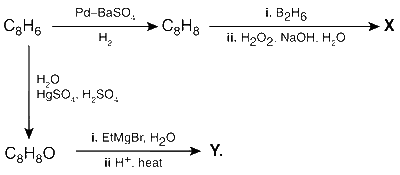
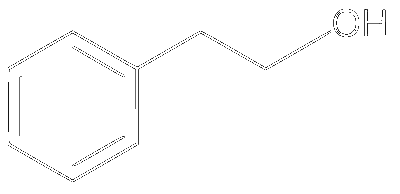
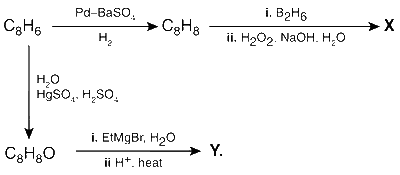
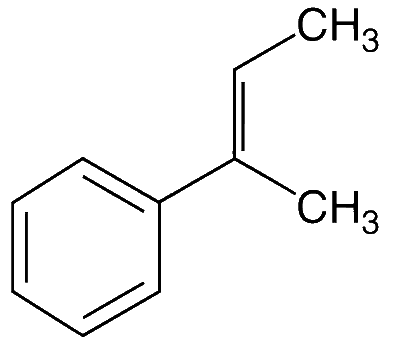
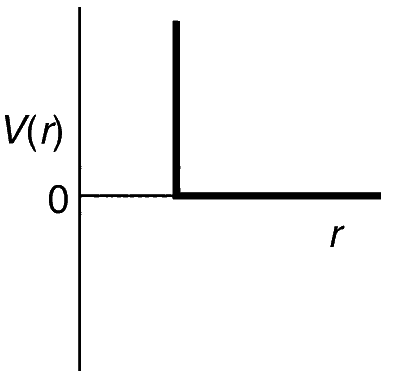JEE Advance - Chemistry (2015 - Paper 2 Offline)
3
The molar conductivity of a solution of a weak acid HX (0.01 M) is 10 times smaller than the molar conductivity of a solution of a weak acid HY (0.10 M). If $$\lambda _{{x^ - }}^0 \approx \lambda _{{y^ - }}^0$$ the difference in their pKa values,
pKa(HX) - pKa(HY), is (consider degree of ionization of both acids to be << 1)
Answer
3
5
Paragraph
When 100 mL of 1.0 M KCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7o C was measured for the beaker and its contents (Expt. 1). Because the enthalpy of neutralization of a strong acid with a strong base is constant (-57.0 kJ/mol), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2) 100 mL of 2.0 M acetic acid (Ka = 2.0 $$\times$$ 10-5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6o C was measured. (Consider heat capacity of all solutions as 4.2 J/gK and density of all solutions as 1.0 g m/L)
Question
The pH of the solution after Expt. 2 is
When 100 mL of 1.0 M KCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7o C was measured for the beaker and its contents (Expt. 1). Because the enthalpy of neutralization of a strong acid with a strong base is constant (-57.0 kJ/mol), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2) 100 mL of 2.0 M acetic acid (Ka = 2.0 $$\times$$ 10-5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6o C was measured. (Consider heat capacity of all solutions as 4.2 J/gK and density of all solutions as 1.0 g m/L)
Question
The pH of the solution after Expt. 2 is
Answer
(B)
4.7
6
Paragraph
When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7o C was measured for the beaker and its contents (Expt. 1). Because the enthalpy of neutralization of a strong acid with a strong base is constant (-57.0 kJ/mol), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2) 100 mL of 2.0 M acetic acid (Ka = 2.0 $$\times$$ 10-5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6o C was measured. (Consider heat capacity of all solutions as 4.2 J/gK and density of all solutions as 1.0 g m/L)
Question
Enthalpy of dissociation (in kJ/mol) of acetic acid obtained from the Expt. 2 is
When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7o C was measured for the beaker and its contents (Expt. 1). Because the enthalpy of neutralization of a strong acid with a strong base is constant (-57.0 kJ/mol), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2) 100 mL of 2.0 M acetic acid (Ka = 2.0 $$\times$$ 10-5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6o C was measured. (Consider heat capacity of all solutions as 4.2 J/gK and density of all solutions as 1.0 g m/L)
Question
Enthalpy of dissociation (in kJ/mol) of acetic acid obtained from the Expt. 2 is
Answer
(A)
1.0