JEE Advance - Chemistry (2015 - Paper 2 Offline - No. 15)
The correct statements regarding (i) HClO, (ii) HClO2, (iii) HClO3 and (iv) HClO4 is (are)
The number of Cl=O bonds (ii) and (iii) together is two.
The number of lone pairs of electrons on Cl in (ii) and (iii) together is three.
The hybridization of Cl in (iv) is sp3
Amongst (i) to (iv), the strongest acid is (i).
Explanation
In all the oxyacids of chlorine : Cl undergoes sp3 hybridization HClO4 is the strongest acid among the given compounds.
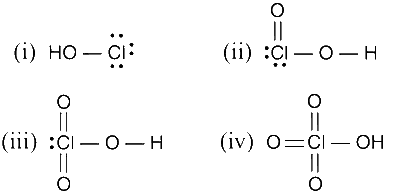
Comments (0)


