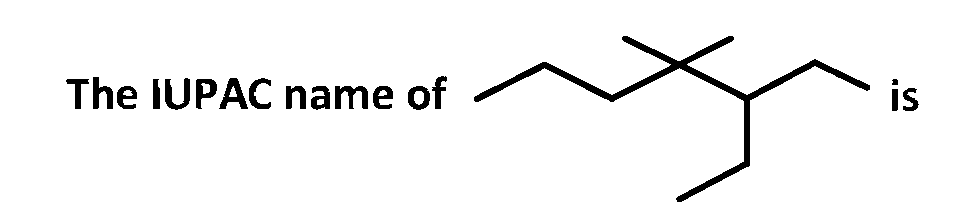
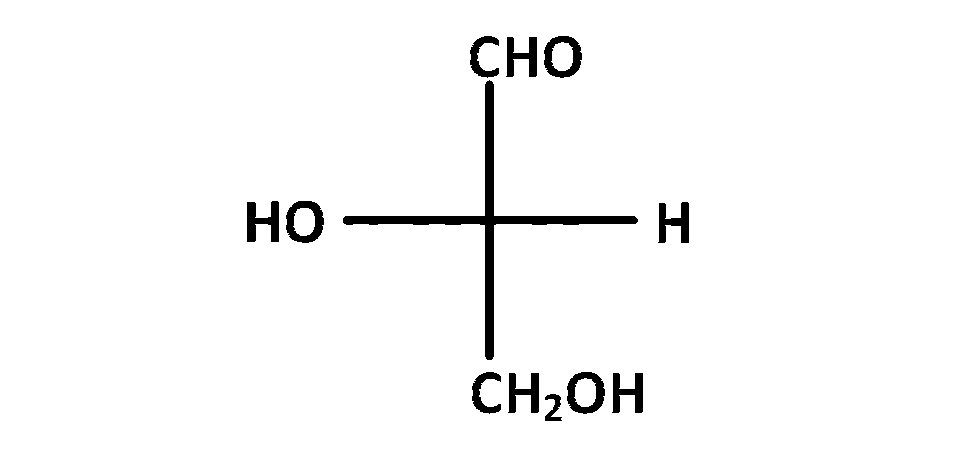
JEE MAIN - Chemistry (2007)
4
In a sautrated solution of the sparingly soluble strong electrolyte AgIO3 (Molecular mass = 283) the
equilibrium which sets in is
AgIO3(s) $$\leftrightharpoons$$ Ag+(aq) + $$IO_3^-$$
If the solubility product constant Ksp of AgIO3 at a given temperature is 1.0 $$\times$$10−8, what is the mass of AgIO3 contained in 100 ml of its saturated solution?
AgIO3(s) $$\leftrightharpoons$$ Ag+(aq) + $$IO_3^-$$
If the solubility product constant Ksp of AgIO3 at a given temperature is 1.0 $$\times$$10−8, what is the mass of AgIO3 contained in 100 ml of its saturated solution?
Answer
(B)
2.83 × 10−3 g
8
In conversion of lime-stone to lime,
CaCO3(s) $$\to$$ CaO(s) + CO2 (g) the vales of ∆H° and ∆S° are +179.1 kJ mol−1 and 160.2 J/K respectively at 298 K and 1 bar. Assuming that ∆H° do not change with temperature, temperature above which conversion of limestone to lime will be spontaneous is :
CaCO3(s) $$\to$$ CaO(s) + CO2 (g) the vales of ∆H° and ∆S° are +179.1 kJ mol−1 and 160.2 J/K respectively at 298 K and 1 bar. Assuming that ∆H° do not change with temperature, temperature above which conversion of limestone to lime will be spontaneous is :
Answer
(D)
1118 K
14
Assuming that water vapour is an ideal gas, the internal energy change $$\left( {\Delta U} \right)$$ when $$1$$ mol of water is vapourised at $$1$$ bar pressure and $${100^ \circ }C$$ (Given : molar enthalpy of vapourisation of water at $$1$$ bar and $$373$$ $$K$$ $$ = 41\,kJ\,mo{l^{ - 1}}\,$$
and $$R = 8.3\,J\,mo{l^{ - 1}}\,{K^{ - 1}}$$ )
and $$R = 8.3\,J\,mo{l^{ - 1}}\,{K^{ - 1}}$$ )
Answer
(D)
$$37.904\,kJ\,mo{l^{ - 1}}$$
18
In the following sequence of reactions,
CH3CH2OH $$\buildrel {P + {I_2}} \over \longrightarrow $$ A $$\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{ether}^{Mg}} $$ B $$\buildrel {HCHO} \over \longrightarrow $$ C $$\buildrel {{H_2}O} \over \longrightarrow $$ D
the compound ‘D’ is
CH3CH2OH $$\buildrel {P + {I_2}} \over \longrightarrow $$ A $$\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{ether}^{Mg}} $$ B $$\buildrel {HCHO} \over \longrightarrow $$ C $$\buildrel {{H_2}O} \over \longrightarrow $$ D
the compound ‘D’ is
Answer
(C)
n-propyl alcohol
26
The energies of activation for forward and reverse reactions for A2 + B2 $$\leftrightharpoons$$ 2AB are 180 kJ mol−1
and 200 kJ mol−1 respectively. The presence of catalyst lowers the activation energy of both (forward and reverse) reactions by 100 kJ mol−1. The enthalpy change of the reaction ( A2 + B2 $$\to$$ 2AB) in the presence of catalyst will be (in kJ mol−1)
Answer
(D)
20
28
The equivalent conductances of two strong electrolytes at infinite dilution in H2O (where ions move
freely through a solution) at 25oC are given below:
$$ \wedge _{C{H_3}COONa}^o$$ = 91.0 S cm2/equiv
$$ \wedge _{HCl}^o$$ = 426.2 S cm2/equiv
What additional information/quantity one needs to calculate $$ \wedge ^o$$ of an aqueous solution of acetic acid?
$$ \wedge _{C{H_3}COONa}^o$$ = 91.0 S cm2/equiv
$$ \wedge _{HCl}^o$$ = 426.2 S cm2/equiv
What additional information/quantity one needs to calculate $$ \wedge ^o$$ of an aqueous solution of acetic acid?
Answer
(B)
$$ \wedge ^o$$ of NaCl