JEE MAIN - Chemistry (2007 - No. 31)
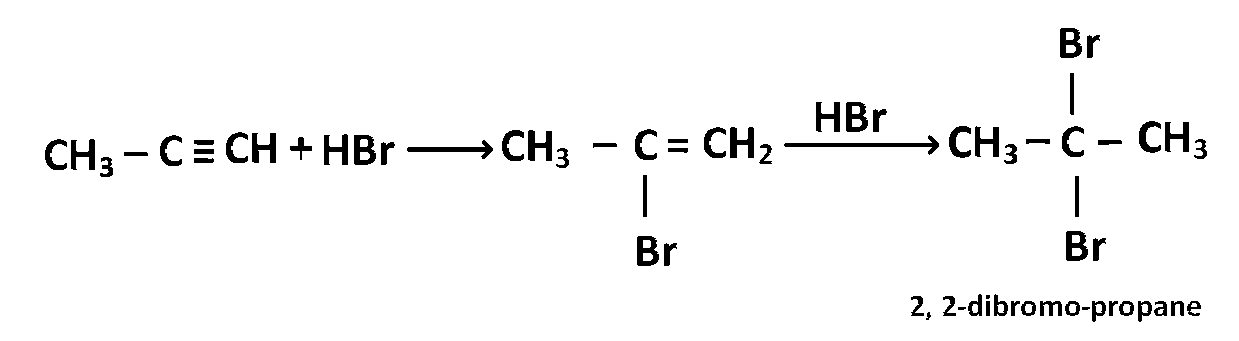
Which of the following reactions will yield 2, 2-dibromopropane?
CH3 - CH = CH2 + HBr $$\to$$
CH3 - C $$\equiv$$ CH + 2HBr $$\to$$
CH3CH = CHBr + HBr $$\to$$
CH $$\equiv$$ CH + 2HBr $$\to$$
Explanation
The reaction follows Markownikoff rule which states that when unsymmetrical reagent adds across unsymmetrical double or triple bond the negative part adds to carbon atom having lesser number of hydrogen atoms.

Comments (0)


