JEE Advance - Physics (2020 - Paper 1 Offline)
1
A small roller of diameter 20 cm has an axle of diameter 10 cm (see figure below on the left). It is
on a horizontal floor and a meter scale is positioned horizontally on its axle with one edge of the scale
on top of the axle (see figure on the right). The scale is now pushed slowly on the axle so that it
moves without slipping on the axle, and the roller starts rolling without slipping. After the roller has
moved 50 cm, the position of the scale will look like (figures are schematic and not drawn to scale)
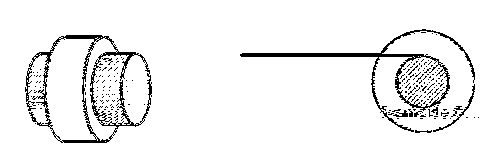
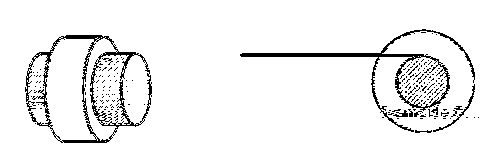
Answer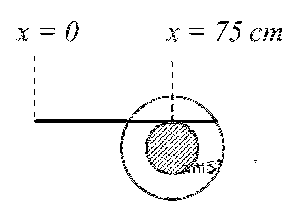
(C)
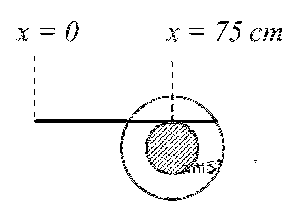
2
A football of radius R is kept on a hole of radius r (r < R) made on a plank kept horizontally. One
end of the plank is now lifted so that it gets tilted making an angle $$\theta $$ from the horizontal as shown in
the figure below. The maximum value of $$\theta $$ so that the football does not start rolling down the plank
satisfies (figure is schematic and not drawn to scale)


Answer
(A)
sin $$\theta $$ = $${r \over R}$$
3
A stationary tuning fork is in resonance with an air column in a pipe. If the tuning fork is moved with
a speed of 2 ms−1
in front of the open end of the pipe and parallel to it, the length of the pipe should
be changed for the resonance to occur with the moving tuning fork. If the speed of sound in air is
320 ms−1, the smallest value of the percentage change required in the length of the pipe is
____________.
Answer
0.62to0.63
4
A circular disc of radius R carries surface charge density
$$\sigma \left( r \right) = {\sigma _0}\left( {1 - {r \over R}} \right)$$, where $$\sigma $$0 is a constant and r is the distance from the center of the disc. Electric flux through a large spherical surface that encloses the charged disc completely is $$\phi $$0. Electric flux through another spherical surface of radius $${R \over 4}$$ and concentric with the disc is $$\phi $$. Then the ratio $${{{\phi _0}} \over \phi }$$ is ________.
$$\sigma \left( r \right) = {\sigma _0}\left( {1 - {r \over R}} \right)$$, where $$\sigma $$0 is a constant and r is the distance from the center of the disc. Electric flux through a large spherical surface that encloses the charged disc completely is $$\phi $$0. Electric flux through another spherical surface of radius $${R \over 4}$$ and concentric with the disc is $$\phi $$. Then the ratio $${{{\phi _0}} \over \phi }$$ is ________.
Answer
6.4
5
As shown schematically in the figure, two vessels contain water solutions (at temperature T) of
potassium permanganate (KMnO4) of different concentrations n1 and n2 (n1 > n2) molecules per
unit volume with $$\Delta $$n = (n1 − n2) << n1. When they are connected by a tube of small length l and
cross-sectional area S, KMnO4 starts to diffuse from the left to the right vessel through the tube.
Consider the collection of molecules to behave as dilute ideal gases and the difference in their partial
pressure in the two vessels causing the diffusion. The speed v of the molecules is limited by the
viscous force −$$\beta $$v on each molecule, where $$\beta $$ is a constant. Neglecting all terms of the order ($$\Delta $$n)2,
which of the following is/are correct? (kB is the Boltzmann constant)


Answer
A
B
C
6
When water is filled carefully in a glass, one can fill it to a height h above the rim of the glass due to
the surface tension of water. To calculate h just before water starts flowing, model the shape of the
water above the rim as a disc of thickness h having semicircular edges, as shown schematically in the
figure. When the pressure of water at the bottom of this disc exceeds what can be withstood due to
the surface tension, the water surface breaks near the rim and water starts flowing from there. If the
density of water, its surface tension and the acceleration
due to gravity are 103 kg m−3 , 0.07 Nm−1 and 10 ms−2 , respectively, the value of h (in mm) is _________.
due to gravity are 103 kg m−3 , 0.07 Nm−1 and 10 ms−2 , respectively, the value of h (in mm) is _________.

Answer
3.74
7
Put a uniform meter scale horizontally on your extended index fingers with the left one at 0.00 cm
and the right one at 90.00 cm. When you attempt to move both the fingers slowly towards the center,
initially only the left finger slips with respect to the scale and the right finger does not. After some
distance, the left finger stops and the right one starts slipping. Then the right finger stops at a distance
xR from the center (50.00 cm) of the scale and the left one starts slipping again. This happens
because of the difference in the frictional forces on the two fingers. If the coefficients of static and
dynamic friction between the fingers and the scale are 0.40 and 0.32, respectively, the value of xR (in
cm) is ______.
Answer
25.60
8
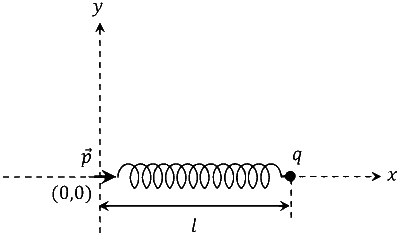
One end of a spring of negligible unstretched length and spring constant k is fixed at the origin (0, 0).
A point particle of mass m carrying a positive charge q is attached at its other end. The entire system
is kept on a smooth horizontal surface. When a point dipole $$\overrightarrow p $$ pointing towards the charge q is fixed
at the origin, the spring gets stretched to a length l and attains a new equilibrium position (see figure
below). If the point mass is now displaced slightly by $$\Delta $$l << l from its equilibrium position and
released, it is found to oscillate at frequency $${1 \over \delta }\sqrt {{k \over m}} $$. The value of $$\delta $$ is ______.

Answer
3.14
9
Consider one mole of helium gas enclosed in a container at initial pressure P1 and volume V1. It
expands isothermally to volume 4V1. After this, the gas expands adiabatically and its volume becomes
32V1. The work done by the gas during isothermal and adiabatic expansion processes are Wiso and
Wadia, respectively. If the ratio $${{{W_{iso}}} \over {{W_{adia}}}}$$ = f ln 2, then f is ______.
Answer
1.77
10
Shown in the figure is a semicircular metallic strip that has thickness t and resistivity $$\rho $$. Its inner
radius is R1 and outer radius is R2. If a voltage V0 is applied between its two ends, a current I flows
in it. In addition, it is observed that a transverse voltage $$\Delta $$V develops between its inner and outer
surfaces due to purely kinetic effects of moving electrons (ignore any role of the magnetic field due
to the current). Then (figure is schematic and not drawn to scale)


Answer
A
C
D
11
A particle of mass m moves in circular orbits with potential energy V(r) = Fr, where F is a positive
constant and r is its distance from the origin. Its energies are calculated using the Bohr model. If the
radius of the particle’s orbit is denoted by R and its speed and energy are denoted by v and E,
respectively, then for the nth orbit (here h is the Planck’s constant)
Answer
B
C
12
Sometimes it is convenient to construct a system of units so that all quantities can be expressed in
terms of only one physical quantity. In one such system, dimensions of different quantities are given
in terms of a quantity X as follows: [position] = [X$$\alpha $$]; [speed] = [X$$\beta $$
]; [acceleration] = [Xp]; [linear
momentum] = [Xq]; [force] = [Xr]. Then
Answer
A
B
13
The filament of a light bulb has surface area 64 mm2
. The filament can be considered as a black
body at temperature 2500 K emitting radiation like a point source when viewed from far. At night
the light bulb is observed from a distance of 100 m. Assume the pupil of the eyes of the observer to
be circular with radius 3 mm. Then
(Take Stefan-Boltzmann constant = 5.67 $$ \times $$ 10−8 Wm−2K−4 , Wien’s displacement constant = 2.90 $$ \times $$ 10−3 m-K, Planck’s constant = 6.63 $$ \times $$ 10−34 Js, speed of light in vacuum = 3.00 $$ \times $$ 108 ms−1)
(Take Stefan-Boltzmann constant = 5.67 $$ \times $$ 10−8 Wm−2K−4 , Wien’s displacement constant = 2.90 $$ \times $$ 10−3 m-K, Planck’s constant = 6.63 $$ \times $$ 10−34 Js, speed of light in vacuum = 3.00 $$ \times $$ 108 ms−1)
Answer
B
C
D
14
An open-ended U-tube of uniform cross-sectional area contains water (density 103 kg m−3
). Initially the
water level stands at 0.29 m from the bottom in each arm. Kerosene oil (a water-immiscible liquid) of
density 800 kg m−3
is added to the left arm until its length is 0.1 m, as shown in the schematic figure
below. The ratio $$\left( {{{{h_1}} \over {{h_2}}}} \right)$$ of the heights of the liquid in the two arms is :


Answer
(B)
$${{35} \over {33}}$$
15
A light disc made of aluminium (a nonmagnetic material) is kept horizontally and is free to rotate
about its axis as shown in the figure. A strong magnet is held vertically at a point above the disc away
from its axis. On revolving the magnet about the axis of the disc, the disc will (figure is schematic
and not drawn to scale)


Answer
(B)
rotate in the same direction as the direction of magnet’s motion
16
A circular coil of radius R and N turns has negligible resistance. As shown in the schematic figure,
its two ends are connected to two wires and it is hanging by those wires with its plane being vertical.
The wires are connected to a capacitor with charge Q through a switch. The coil is in a horizontal
uniform magnetic field Bo parallel to the plane of the coil. When the switch is closed, the capacitor
gets discharged through the coil in a very short time. By the time the capacitor is discharged fully,
magnitude of the angular momentum gained by the coil will be (assume that the discharge time is so
short that the coil has hardly rotated during this time)


Answer
(B)
$$\pi NQ{B_0}{R^2}$$
18
A uniform electric field, $$\overrightarrow E = - 400\sqrt 3 \widehat y$$ NC−1
is applied in a region. A charged particle of mass m
carrying positive charge q is projected in this region with an initial speed of 2$$\sqrt {10} $$ $$ \times $$ 106 ms−1
. This
particle is aimed to hit a target T, which is 5 m away from its entry point into the field as shown
schematically in the figure.
Take $${q \over m}$$ = 1010 Ckg−1 . Then
Take $${q \over m}$$ = 1010 Ckg−1 . Then

Answer
B
C



