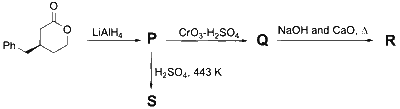
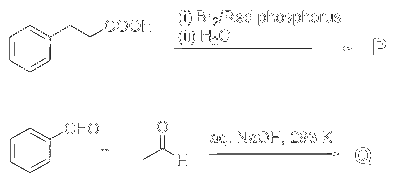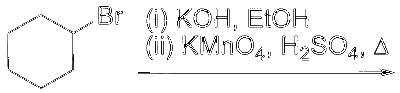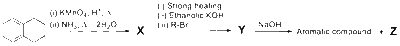JEE Advance - Chemistry (2025 - Paper 2 Online)
The solubility of barium iodate in an aqueous solution prepared by mixing 200 mL of 0.010 M barium nitrate with 100 mL of 0.10 M sodium iodate is $\boldsymbol{X} \times 10^{-6} \mathrm{~mol} \mathrm{dm}^{-3}$. The value of $\boldsymbol{X}$ is ____________.
Use: Solubility product constant $\left(K_{\mathrm{sp}}\right)$ of barium iodate $=1.58 \times 10^{-9}$
Adsorption of phenol from its aqueous solution on to fly ash obeys Freundlich isotherm. At a given temperature, from $10 \mathrm{mg} \mathrm{g}^{-1}$ and $16 \mathrm{mg} \mathrm{g}^{-1}$ aqueous phenol solutions, the concentrations of adsorbed phenol are measured to be $4 \mathrm{mg} \mathrm{g}^{-1}$ and $10 \mathrm{mg} \mathrm{g}^{-1}$, respectively. At this temperature, the concentration (in $\mathrm{mg} \mathrm{g}^{-1}$ ) of adsorbed phenol from $20 \mathrm{mg} \mathrm{g}^{-1}$ aqueous solution of phenol will be ______________.
Use: $\log _{10} 2=0.3$
Consider a reaction $A+R \rightarrow$ Product. The rate of this reaction is measured to be $k[A][R]$. At the start of the reaction, the concentration of $R,[R]_0$, is 10-times the concentration of $A,[A]_0$. The reaction can be considered to be a pseudo first order reaction with assumption that $k[R]=k^{\prime}$ is constant. Due to this assumption, the relative error (in %) in the rate when this reaction is $40 \%$ complete, is ___________.
[ $k$ and $k^{\prime}$ represent corresponding rate constants]
At 300 K , an ideal dilute solution of a macromolecule exerts osmotic pressure that is expressed in terms of the height $(h)$ of the solution (density $=1.00 \mathrm{~g} \mathrm{~cm}^{-3}$ ) where $h$ is equal to 2.00 cm . If the concentration of the dilute solution of the macromolecule is $2.00 \mathrm{~g} \mathrm{dm}^{-3}$, the molar mass of the macromolecule is calculated to be $\boldsymbol{X} \times 10^4 \mathrm{~g} \mathrm{~mol}^{-1}$. The value of $\boldsymbol{X}$ is __________.
Use: Universal gas constant $(R)=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$ and acceleration due to gravity $(g)=10 \mathrm{~m} \mathrm{~s}^{-2}$
An electrochemical cell is fueled by the combustion of butane at 1 bar and 298 K . Its cell potential is $\frac{\boldsymbol{X}}{F} \times 10^3$ volts, where $F$ is the Faraday constant. The value of $\boldsymbol{X}$ is _____________.
Use: Standard Gibbs energies of formation at 298 K are: $\Delta_f G_{\mathrm{CO}_2}^o=-394 \mathrm{~kJ} \mathrm{~mol}^{-1} ; \Delta_f G_{\text {water }}^o=$ $-237 \mathrm{~kJ} \mathrm{~mol}^{-1} ; \Delta_f G_{\text {butane }}^o=-18 \mathrm{~kJ} \mathrm{~mol}^{-1}$
A linear octasaccharide (molar mass $=1024 \mathrm{~g} \mathrm{~mol}^{-1}$ ) on complete hydrolysis produces three monosaccharides: ribose, 2-deoxyribose and glucose. The amount of 2-deoxyribose formed is $58.26 \%(\mathrm{w} / \mathrm{w})$ of the total amount of the monosaccharides produced in the hydrolyzed products. The number of ribose unit(s) present in one molecule of octasaccharide is $\qquad$ .
Use: Molar mass $\left(\right.$ in g $\left.\mathrm{mol}^{-1}\right)$ : ribose $=150,2$-deoxyribose $=134$, glucose $=180$;
Atomic mass (in amu): $\mathrm{H}=1, \mathrm{O}=16$