WAEC - Chemistry (2021 - No. 42)
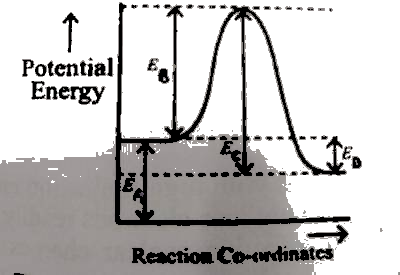
The enthalpy change of the reaction is------------
E\(_{A}\)
E\(_{B}\)
E\(_{C}\)
E\(_{D}\)
Explanation
Enthalpy change, ΔH = ΔH\(_{product}\) - ΔH\(_{reactant}\)
From the diagram E\(_D\) represents the enthalpy change, ΔH.
Comments (0)



