WAEC - Chemistry (1993 - No. 1)
Copper metal will react with concentrated trioxonitrate (V) acid to give?
Cu(NO3)3 + NO + N2O4 + H2O
Cu(NO3)2 + NO + H2O
CuO + NO2 + H2O
Cu(NO3)2 + 2NO2 + 2H2O
Explanation
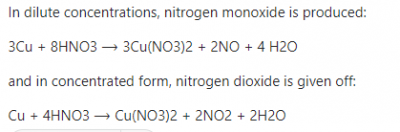
Generally, when metals react with acids, Hydrogen gas is usually liberated, with an exception of dilute Trioxonitrate(V) acids. However, in this case, it is not the usual Acid- metal reaction, as Copper metal is reacting with concentrated nitric acid. Thus, Copper is oxidized by the concentrated nitric acid, HNO3, to produce Cu2+ ions while the nitric acid is reduced to nitrogen dioxide, NO2 and water is also formed. Therefore, the correct answer is option D.
Comments (0)


