JEE Advance - Physics (2025 - Paper 2 Online - No. 13)
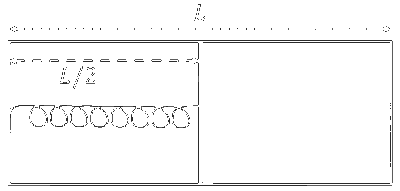
Explanation
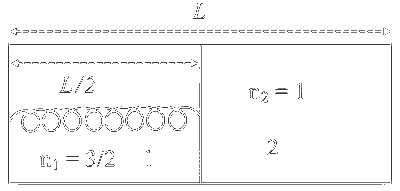
Extension in spring
$$ \begin{aligned} & \mathrm{x}=0.5 \mathrm{~L}-0.4 \mathrm{~L} \\ & =0.1 \mathrm{~L} \end{aligned} $$
FBD of piston
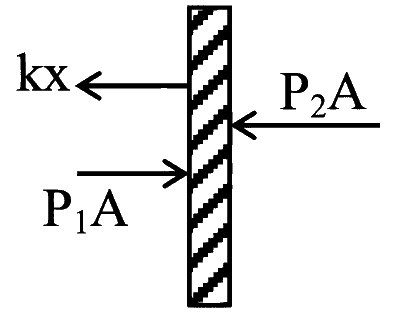
$$ \begin{aligned} & \mathrm{kx}+\mathrm{P}_2 \mathrm{~A}=\mathrm{P}_1 \mathrm{~A} \\ & \mathrm{P}_2 \mathrm{~A}=\mathrm{P}_1 \mathrm{~A}-\mathrm{kx} \\ & \mathrm{P}_2=\mathrm{P}_1-\frac{\mathrm{kL}}{\mathrm{~A}(10)} ........(i) \\ & \mathrm{P}_1 \mathrm{~V}=\mathrm{n}_1 \mathrm{RT} \\ & \mathrm{P}_2 \mathrm{~V}=\mathrm{n}_2 \mathrm{RT} \\ & \frac{\mathrm{P}_1}{\mathrm{P}_2}=\frac{\mathrm{n}_1}{\mathrm{n}_2}=\frac{3}{2} \end{aligned} $$
$$ \begin{aligned} & \mathrm{P}_1=\frac{3}{2} \mathrm{P}_2 ........(ii) \\ & \mathrm{P}_2=\frac{3}{2} \mathrm{P}_2-\frac{\mathrm{kL}}{10 \mathrm{~A}} \\ & \frac{\mathrm{P}_2}{2}=\frac{\mathrm{kL}}{10 \mathrm{~A}} \\ & \mathrm{P}_2=\frac{\mathrm{kL}}{5 \mathrm{~A}}=\frac{\mathrm{kL}}{\mathrm{~A}} \alpha \\ & \alpha=\frac{1}{5}=0.2 \end{aligned} $$
Comments (0)


