JEE Advance - Physics (2020 - Paper 2 Offline - No. 5)
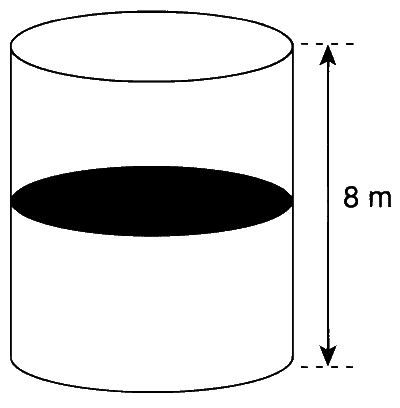
A thermally isolated cylindrical closed vessel of height 8 m is kept vertically. It is divided into two
equal parts by a diathermic (perfect thermal conductor) frictionless partition of mass 8.3 kg. Thus the
partition is held initially at a distance of 4 m from the top, as shown in the schematic figure below.
Each of the two parts of the vessel contains 0.1 mole of an ideal gas at temperature 300 K. The
partition is now released and moves without any gas leaking from one part of the vessel to the other.
When equilibrium is reached, the distance of the partition from the top (in m) will be _______.
(take the acceleration due to gravity = 10 ms−2 and the universal gas constant = 8.3 J mol−1K−1).
(take the acceleration due to gravity = 10 ms−2 and the universal gas constant = 8.3 J mol−1K−1).

Answer
6
Explanation
To start with, both the compartments have same volume. Under the action of weight, moving down by distance $x$, length of upper compartment will be $(4+x)$ and that of lower compartment $(4-x)$.
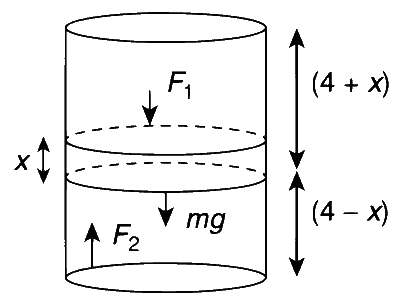
Volumes of two compartments are
$$ V_1=(4+x) A \text { and } V_2=(4-x) A $$
At equilibrium,
$$ F_2=F_1+m g $$
$$ \begin{aligned} & P_2 A=P_1 A+m g \\\\ & P_2=P_1+\frac{m g}{A} \end{aligned} $$
From $P V=n R T$, we get $P=\frac{n R T}{V}$
$$ \begin{aligned} & \frac{n R T}{V_2}=\frac{n R T}{V_1}+\frac{m g}{A} \\\\ \Rightarrow & n R T\left[\frac{1}{V_2}-\frac{1}{V_1}\right]=\frac{m g}{A} \\\\ \Rightarrow & n R T\left[\frac{1}{A(4-x)}-\frac{1}{4(4+x)}\right]=\frac{m g}{A} \\\\ \Rightarrow & \frac{n R T}{A}\left[\frac{(4+x)-(4-x)}{(4-x)(4+x)}\right]=\frac{m g}{A} \\\\ \Rightarrow & n R T\left[\frac{2 x}{\left(16-x^2\right)}\right]=m g \\\\ \Rightarrow & 0.1 \times 8.3 \times 300\left[\frac{2 x}{\left(16-x^2\right)}\right]=8.3 \times 10 \\\\ \Rightarrow & \frac{6 x}{16-x^2}=1 \Rightarrow 16-x^2=6 x \\\\ \Rightarrow & x^2+6 x-16=0 \\\\ \Rightarrow & x=\frac{-6 \pm \sqrt{36+64}}{2} \\\\ & x=\frac{-6 \pm \sqrt{100}}{2}=\frac{-6 \pm 10}{2} \\\\ & x=\frac{10-6}{2} \text { or } \frac{-10-6}{2}=-8 \text { or } 2 \end{aligned} $$
Neglecting negative $\operatorname{sign} x=2$
Partition from top $=4+2=6 \mathrm{~m}$

Volumes of two compartments are
$$ V_1=(4+x) A \text { and } V_2=(4-x) A $$
At equilibrium,
$$ F_2=F_1+m g $$
$$ \begin{aligned} & P_2 A=P_1 A+m g \\\\ & P_2=P_1+\frac{m g}{A} \end{aligned} $$
From $P V=n R T$, we get $P=\frac{n R T}{V}$
$$ \begin{aligned} & \frac{n R T}{V_2}=\frac{n R T}{V_1}+\frac{m g}{A} \\\\ \Rightarrow & n R T\left[\frac{1}{V_2}-\frac{1}{V_1}\right]=\frac{m g}{A} \\\\ \Rightarrow & n R T\left[\frac{1}{A(4-x)}-\frac{1}{4(4+x)}\right]=\frac{m g}{A} \\\\ \Rightarrow & \frac{n R T}{A}\left[\frac{(4+x)-(4-x)}{(4-x)(4+x)}\right]=\frac{m g}{A} \\\\ \Rightarrow & n R T\left[\frac{2 x}{\left(16-x^2\right)}\right]=m g \\\\ \Rightarrow & 0.1 \times 8.3 \times 300\left[\frac{2 x}{\left(16-x^2\right)}\right]=8.3 \times 10 \\\\ \Rightarrow & \frac{6 x}{16-x^2}=1 \Rightarrow 16-x^2=6 x \\\\ \Rightarrow & x^2+6 x-16=0 \\\\ \Rightarrow & x=\frac{-6 \pm \sqrt{36+64}}{2} \\\\ & x=\frac{-6 \pm \sqrt{100}}{2}=\frac{-6 \pm 10}{2} \\\\ & x=\frac{10-6}{2} \text { or } \frac{-10-6}{2}=-8 \text { or } 2 \end{aligned} $$
Neglecting negative $\operatorname{sign} x=2$
Partition from top $=4+2=6 \mathrm{~m}$
Comments (0)


