JEE Advance - Physics (2019 - Paper 2 Offline - No. 18)
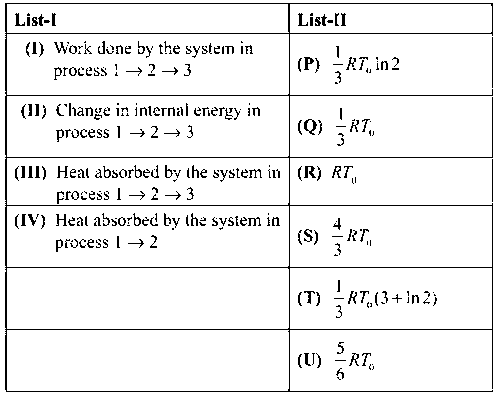
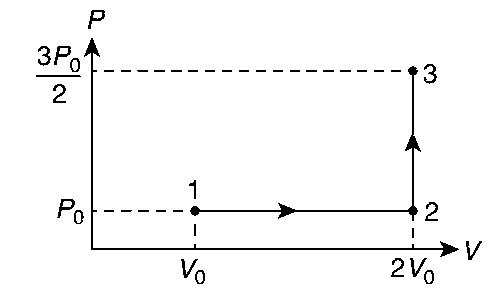
If the process carried out on one mole of monoatomic ideal gas is as shown in the PV-diagram with $${p_0}{V_0} = {1 \over 3}R{T_0}$$, the correct match is,

I$$ \to $$S, II $$ \to $$ R, III $$ \to $$ Q, IV $$ \to $$ T
I $$ \to $$ Q, II $$ \to $$ R, III $$ \to $$ P, IV $$ \to $$ U
I $$ \to $$ Q, II $$ \to $$ S, III $$ \to $$ R, IV $$ \to $$ U
I $$ \to $$ Q, II $$ \to $$ R, III $$ \to $$ S, IV $$ \to $$ U
Explanation
(I) $${W_{1 \to 2 \to 3}} = {W_{1 \to 2}} + {W_{2 \to 3}} = {P_0}[2{V_0} - {V_0}] + 0 = {P_0}{V_0}$$
$${W_{1 \to 2 \to 3}} = {P_0}{V_0} = {{R{T_0}} \over 3}$$ (I$$ \to $$Q)
$${U_{1 \to 2 \to 3}} = {3 \over 2}\left[ {{{3{P_0}} \over 2} \times 2{V_0} - {P_0}{V_0}} \right] = {3 \over 2} \times 2{P_0}{V_0} = 3{P_0}{V_0} = R{T_0}$$(II$$ \to $$R)
(III) $${Q_{1 \to 2 \to 3}} = {U_{1 \to 2 \to 3}} + {W_{1 \to 2 \to 3}} = R{T_0} + {{R{T_0}} \over 3} = {{4R{T_0}} \over 3}$$(III$$ \to $$S)
(IV) $${Q_{1 \to 2}} = n{C_p}\Delta T = n{5 \over 2}R({T_2} - {T_1}) = {5 \over 2}[{P_0}2{V_0} - {P_0}{V_0}] = {5 \over 2}{P_0}{V_0} = {5 \over 2}{{R{T_0}} \over 3} = {5 \over 6}{R_0}{T_0}$$ (IV$$ \to $$U)
$${W_{1 \to 2 \to 3}} = {P_0}{V_0} = {{R{T_0}} \over 3}$$ (I$$ \to $$Q)
$${U_{1 \to 2 \to 3}} = {3 \over 2}\left[ {{{3{P_0}} \over 2} \times 2{V_0} - {P_0}{V_0}} \right] = {3 \over 2} \times 2{P_0}{V_0} = 3{P_0}{V_0} = R{T_0}$$(II$$ \to $$R)
(III) $${Q_{1 \to 2 \to 3}} = {U_{1 \to 2 \to 3}} + {W_{1 \to 2 \to 3}} = R{T_0} + {{R{T_0}} \over 3} = {{4R{T_0}} \over 3}$$(III$$ \to $$S)
(IV) $${Q_{1 \to 2}} = n{C_p}\Delta T = n{5 \over 2}R({T_2} - {T_1}) = {5 \over 2}[{P_0}2{V_0} - {P_0}{V_0}] = {5 \over 2}{P_0}{V_0} = {5 \over 2}{{R{T_0}} \over 3} = {5 \over 6}{R_0}{T_0}$$ (IV$$ \to $$U)
Comments (0)