JEE Advance - Physics (2017 - Paper 1 Offline - No. 15)
Which one of the following options is the correct combination?
$$\left( {{\rm I}V} \right)\left( {ii} \right)\left( S \right)$$
$$\left( {{\rm I}{\rm I}{\rm I}} \right)\left( {ii} \right)\left( S \right)$$
$$\left( {{\rm I}{\rm I}} \right)\left( {iv} \right)\left( P \right)$$
$$\left( {{\rm I}{\rm I}} \right)\left( {iv} \right)\left( R \right)$$
Explanation
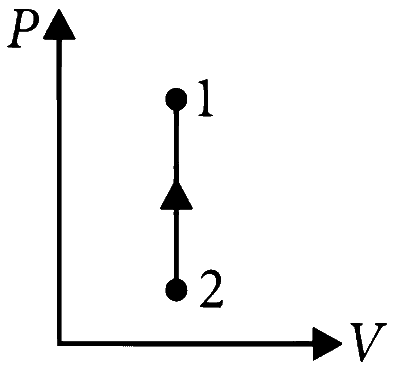
$$\because$$ dW = PdV
and there is no change in volume in isochoric process.
$$\therefore$$ dW = 0
Comments (0)




