JEE Advance - Physics (2014 - Paper 1 Offline - No. 20)
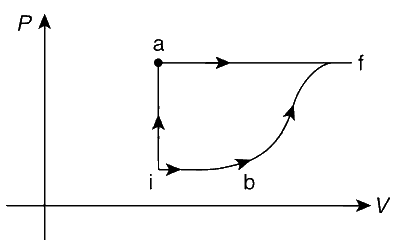
A thermodynamic system is taken from an initial state i with internal energy Ui = 100 J to the final state f along two different paths iaf and ibf, as schematically shown in the figure. The work done by the system along the paths af, ib and bf are Waf = 200 J, Wib = 50 J and Wbf = 100 J respectively. The heat supplied to the system along the path iaf, ib and bf are Qiaf, Qib and Qbf respectively. If the internal energy of the system in the state b is Ub = 200 J and Qiaf = 500 J, the ratio Qbf / Qib is

Answer
2
Explanation
In a thermodynamics process, the heat supplied to the system, the increase in internal energy of the system, and the work done by the system are related by the first law of thermodynamics, $$\Delta Q = \Delta U + \Delta W$$.
The first law of thermodynamics for the process iaf gives
$${Q_{iaf}} = {U_{iaf}} + {W_{iaf}} = ({U_f} - {U_i}) + ({W_{ia}} + {W_{af}})$$ ..... (1)
Substitute Qiaf = 500 J, Ui = 100 J, Wia = 0 (constant volume), and Waf = 200 J in equation (1) to get Uf = 400 J.
In the process ib,
$${Q_{ib}} = {U_{ib}} + {W_{ib}} = ({U_b} - {U_i}) + {W_{ib}}$$ ..... (2)
Substitute Ub = 200 J, Ui = 100 J, and Wib = 50 J in equation (2) to get Qib = 150 J.
In the process bf,
$${Q_{bf}} = {U_{bf}} + {W_{bf}} = ({U_f} - {U_b}) + {W_{bf}}$$ ..... (3)
Substitute Uf = 400 J, Ub = 200 J and Wbf = 100 J in equation (3) to get Qbf = 300 J. Thus, Qbf/Qib = 300/150 = 2.
The first law of thermodynamics for the process iaf gives
$${Q_{iaf}} = {U_{iaf}} + {W_{iaf}} = ({U_f} - {U_i}) + ({W_{ia}} + {W_{af}})$$ ..... (1)
Substitute Qiaf = 500 J, Ui = 100 J, Wia = 0 (constant volume), and Waf = 200 J in equation (1) to get Uf = 400 J.
In the process ib,
$${Q_{ib}} = {U_{ib}} + {W_{ib}} = ({U_b} - {U_i}) + {W_{ib}}$$ ..... (2)
Substitute Ub = 200 J, Ui = 100 J, and Wib = 50 J in equation (2) to get Qib = 150 J.
In the process bf,
$${Q_{bf}} = {U_{bf}} + {W_{bf}} = ({U_f} - {U_b}) + {W_{bf}}$$ ..... (3)
Substitute Uf = 400 J, Ub = 200 J and Wbf = 100 J in equation (3) to get Qbf = 300 J. Thus, Qbf/Qib = 300/150 = 2.
Comments (0)


