JEE Advance - Physics (2013 - Paper 2 Offline - No. 10)
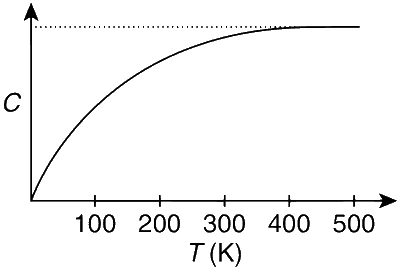
The figure below shows the variation of specific heat capacity (C) of a solid as a function of temperature (T). The temperature is increased continuously from 0 to 500 K at a constant rate. Ignoring any volume change, the following statement(s) is(are) correct to a reasonable approximation.
Explanation

According to definition of specific heat capacity,
$$C = {{\Delta Q} \over {m\Delta T}}$$
$$ \Rightarrow \Delta Q = mC\Delta T$$ $$\therefore$$ $${{\Delta Q} \over {\Delta t}} = mC{{\Delta T} \over {\Delta t}}$$
Rate of heat absorbed $$R = {{\Delta Q} \over {\Delta t}}$$
$$ \Rightarrow {{\Delta Q} \over {\Delta t}} \propto C$$
(a) In 0-100 K,
C increases with T but not linearly. So R increases but not linearly.
(b) As $$\Delta Q = mC\Delta T$$
$$Q = m\int {C\Delta T} $$
= m area under C-T curve
From the graph it is clear that area under C-T is more in 400-500 K than in 0-100 K.
Therefore, heat absorbed in 0-100 K is less than in 400-500 K.
(c) In 400-500 K,
C remains constant so there is no change in R.
(d) In 200-300 K,
C increases so R increases.
Comments (0)


