JEE Advance - Physics (2010 - Paper 1 Offline - No. 17)
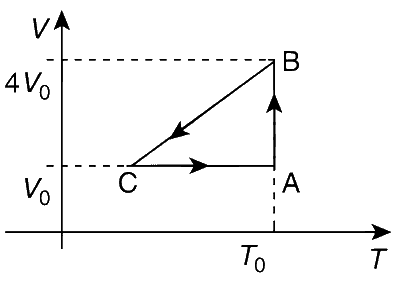
One mole of an ideal gas in initial state A undergoes a cyclic process ABCA, as shown in the figure. Its pressure at A is P0. Choose the correct option(s) from the following:
Explanation
The internal energy of one mole of an ideal gas at temperature T is given by U = 3RT/2. The process AB is isothermal i.e., TA = TB = T0, which makes UA = UB.
The work done in the isothermal process AB is
WAB = nRT ln(VB/VA)
= nRT0 ln(4V0/V0) = p0V0 ln4.
Information regarding p and T at C can not be obtained from the given graph. Unless it is mentioned that line BC passes through origin or not.
Hence, the correct options are (a) and (b).
Note:
If we assume that the line BC pass through the origin. In the process BC, the slope V/T is constant. Thus, BC is an isobaric process (as V/T = nR/p, by the ideal gas equation). Thus,
pC = pB = RTB / VB
= RT0/(4V0) = (RT0/V0)4 = p0/4.
Apply the ideal gas equation for the states A and C to get
$${T_C} = {{{p_C}{V_C}} \over {{p_0}{V_0}}}{T_0} = {{({p_0}/4){V_0}} \over {{p_0}{V_0}}}{T_0} = {T_0}/4$$.
Comments (0)


