JEE Advance - Physics (2009 - Paper 2 Offline - No. 5)
The figure shows the PV plot of an ideal gas taken through a cycle ABCDA. The part ABC is a semicircle and CDA is half of an ellipse. Then,
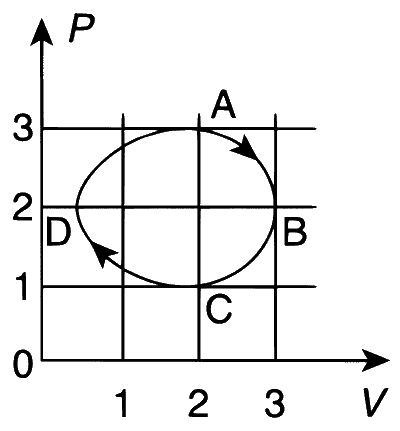
the process during the path A $$\to$$ B is isothermal.
heat flows out of the gas during the path B $$\to$$ C $$\to$$ D.
work done during the path A $$\to$$ B $$\to$$ C is zero.
positive work is done by the gas in the cycle ABCDA.
Explanation
We observe the following:
$$\bullet$$ For the path B $$\to$$ C $$\to$$ D, the volume (V) decreases; $$\Delta$$W < 0.
$$\Delta U < 0 \Rightarrow \Delta Q < 0$$
$$\bullet$$ For the process A $$\to$$ B $$\to$$ C, $$\Delta$$W is the area of the semicircle and $$\Delta W \ne 0$$.
$$\bullet$$ For the process A $$\to$$ B (semicircle), it cannot be an isothermal process (whose PV-diagram is a rectangular hyperbola).
$$\bullet$$ For (clockwise) process A $$\to$$ B $$\to$$ C $$\to$$ D $$\to$$ A, $$\Delta$$W is the area enclosed, which is positive.
Comments (0)


