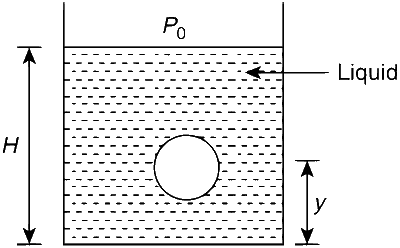
JEE Advance - Physics (2008 - Paper 1 Offline - No. 17)
Explanation
Buoyancy force = Weight of fluid displaced
= (mass of fluid displaced)g
$$ = V{\rho _l}g$$ .... (i)
Where V = Volume of fluid displaced
= Volume of the bubble
$$PV = nRT$$
$$ \Rightarrow V = {{nRT} \over P} = {{nRT} \over {{P_0}(H - y){\rho _l}g}}$$ .... (ii)
Where P is the pressure of the bubble at an arbitrary location at a distance 'y' from the bottom.
Put the value of temperature from eq. (i)
$$V = {{nR} \over {[{P_0} + (H - y){\rho _l}g]}} \times {{{T_0}{{[{P_0} + (H - y){\rho _l}g]}^{2/5}}} \over {{{[{P_0} + H{\rho _l}g]}^{2/5}}}}$$
$$ = {{nR{T_0}} \over {{{[{P_0} + (H - y){\rho _l}g]}^{3/5}}{{[{P_0} + H{\rho _l}g]}^{2/5}}}}$$ .... (iii)
From eq. (i) and (iii) Buoyance force
$$ = {{nR{T_0}{\rho _1}g} \over {{{[{P_0} + (H - y){\rho _l}g]}^{3/5}}{{[{P_0} + H{\rho _l}g]}^{2/5}}}}$$
Comments (0)