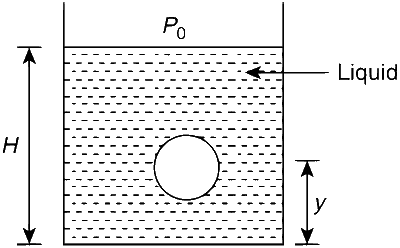
JEE Advance - Physics (2008 - Paper 1 Offline - No. 16)
When the gas bubble is at a height y from the bottom, its temperature is :
$${T_0}{\left( {{{{P_0} + {\rho _l}gH} \over {{P_0} + {\rho _l}gy}}} \right)^{{2 \over 5}}}$$
$${T_0}{\left( {{{{P_0} + {\rho _l}g(H - y)} \over {{P_0} + {\rho _l}gH}}} \right)^{{2 \over 5}}}$$
$${T_0}{\left( {{{{P_0} + {\rho _l}gH} \over {{P_0} + {\rho _l}gy}}} \right)^{{3 \over 5}}}$$
$${T_0}{\left( {{{{P_0} + {\rho _l}g(H - y)} \over {{P_0} + {\rho _l}gH}}} \right)^{{3 \over 5}}}$$
Explanation
Since the process is adiabatic,
$$P{V^\gamma }$$ = constant for gas inside bubble.
Thus, $$P{V^{(1 - \gamma )}}.\,{T^\gamma } = $$ constant
$$ \Rightarrow {P^{1 - \gamma }}_{bottom}{T^\gamma }_{bottom} = P_y^{1 - \gamma }T_y^\gamma = $$ constant
$$ \Rightarrow {({P_0} + {\rho _1}gH)^{ - 2/3}}(T_0^{5/3}) = ({P_0} + {\rho _1}g{(H - y)^{ - 2/3}}){T^{5/3}}$$
$$ \Rightarrow {T_0} = {\left[ {{{{P_0} + {\rho _1}g(H - y)} \over {{P_0} + {\rho _1}gH}}} \right]^{2/5}}$$
Comments (0)