JEE Advance - Chemistry (2024 - Paper 2 Online - No. 14)
An organic compound $\mathbf{P}$ with molecular formula $\mathrm{C}_9 \mathrm{H}_{18} \mathrm{O}_2$ decolorizes bromine water and also shows positive iodoform test. $\mathbf{P}$ on ozonolysis followed by treatment with $\mathrm{H}_2 \mathrm{O}_2$ gives $\mathbf{Q}$ and $\mathbf{R}$. While compound $\mathbf{Q}$ shows positive iodoform test, compound $\mathbf{R}$ does not give positive iodoform test. $\mathbf{Q}$ and $\mathbf{R}$ on oxidation with pyridinium chlorochromate (PCC) followed by heating give $\mathbf{S}$ and $\mathbf{T}$, respectively. Both $\mathbf{S}$ and $\mathbf{T}$ show positive iodoform test.
Complete copolymerization of 500 moles of $\mathbf{Q}$ and 500 moles of $\mathbf{R}$ gives one mole of a single acyclic copolymer $\mathbf{U}$.
[Given, atomic mass: $\mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16$ ]
An organic compound $\mathbf{P}$ with molecular formula $\mathrm{C}_9 \mathrm{H}_{18} \mathrm{O}_2$ decolorizes bromine water and also shows positive iodoform test. $\mathbf{P}$ on ozonolysis followed by treatment with $\mathrm{H}_2 \mathrm{O}_2$ gives $\mathbf{Q}$ and $\mathbf{R}$. While compound $\mathbf{Q}$ shows positive iodoform test, compound $\mathbf{R}$ does not give positive iodoform test. $\mathbf{Q}$ and $\mathbf{R}$ on oxidation with pyridinium chlorochromate (PCC) followed by heating give $\mathbf{S}$ and $\mathbf{T}$, respectively. Both $\mathbf{S}$ and $\mathbf{T}$ show positive iodoform test.
Complete copolymerization of 500 moles of $\mathbf{Q}$ and 500 moles of $\mathbf{R}$ gives one mole of a single acyclic copolymer $\mathbf{U}$.
[Given, atomic mass: $\mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16$ ]
An organic compound $\mathbf{P}$ with molecular formula $\mathrm{C}_9 \mathrm{H}_{18} \mathrm{O}_2$ decolorizes bromine water and also shows positive iodoform test. $\mathbf{P}$ on ozonolysis followed by treatment with $\mathrm{H}_2 \mathrm{O}_2$ gives $\mathbf{Q}$ and $\mathbf{R}$. While compound $\mathbf{Q}$ shows positive iodoform test, compound $\mathbf{R}$ does not give positive iodoform test. $\mathbf{Q}$ and $\mathbf{R}$ on oxidation with pyridinium chlorochromate (PCC) followed by heating give $\mathbf{S}$ and $\mathbf{T}$, respectively. Both $\mathbf{S}$ and $\mathbf{T}$ show positive iodoform test.
Complete copolymerization of 500 moles of $\mathbf{Q}$ and 500 moles of $\mathbf{R}$ gives one mole of a single acyclic copolymer $\mathbf{U}$.
[Given, atomic mass: $\mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16$ ]
Explanation
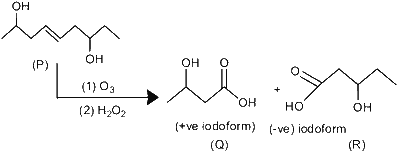
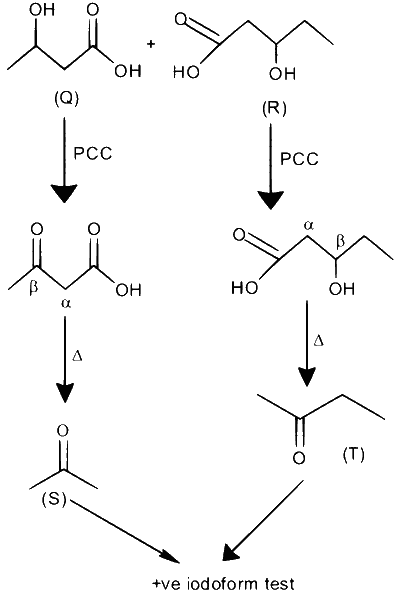
$\therefore$ Sum of oxygen atoms in $\mathrm{S}$ and $\mathrm{T}=1+1=2$
Comments (0)


