JEE Advance - Chemistry (2024 - Paper 2 Online - No. 12)
Explanation
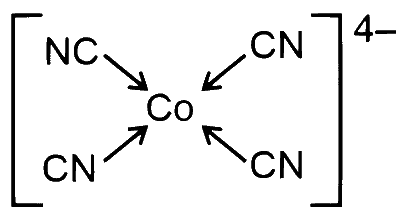
$$ [\mathrm{Co}(\mathrm{CN})_4]^{4-} \Rightarrow \mathrm{Co}^0 \Rightarrow 3 d^7 4 s^2 $$
Due to SFL, $\mathrm{CN}^{-}$pairing and transference of electron takes place and hybridisation is $d s p^2$
Geometry $\Rightarrow$ Square planer
OR
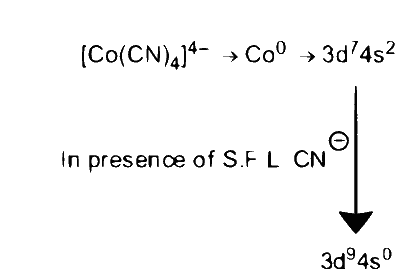
$\begin{gathered}\Rightarrow \text { hybridisation }\left[\mathrm{Co}(\mathrm{CN})_4\right]^{4-} \end{gathered}$ $$ \to $$ $\mathrm{sp}^3$
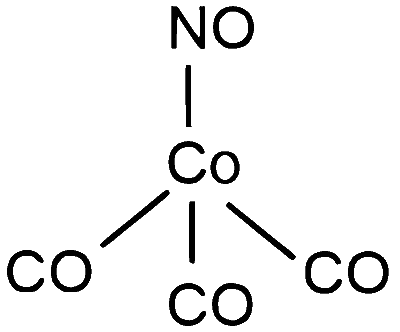
$\begin{aligned} & {\left[\mathrm{Co}(\mathrm{CO})_3(\mathrm{NO})\right] \rightarrow \mathrm{Co}^{-1} \rightarrow 3\mathrm{d}^8 4 \mathrm{s}^2 \rightarrow 3 \mathrm{d}^{10} \text { ( In presence of S.F.L) }} \\\\ & \quad \text { here NO present as }+1 \text { state } \\\\ & \Rightarrow \text { Hybridisation } \rightarrow\left[\mathrm{Co}(\mathrm{CO})_3(\mathrm{NO})\right] \rightarrow \mathrm{sp}^3\end{aligned}$
Geometry $=$ Tetrahedral
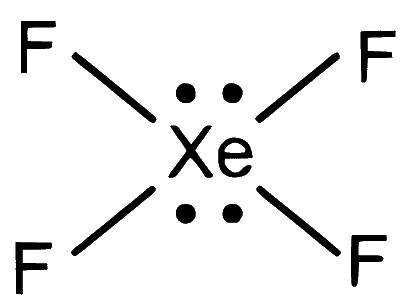
$$ \mathrm{XeF}_4 \Rightarrow 4 \mathrm{bp}+2 lp \Rightarrow s p^3 d^2 $$
Geometry = Square planer
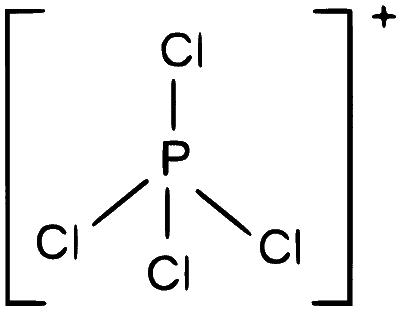
$$ \mathrm{PCl}_4^{+} \Rightarrow 4 \mathrm{lb}+0 \mathrm{lp} $$
$s p^3 \Rightarrow$ tetrahedral
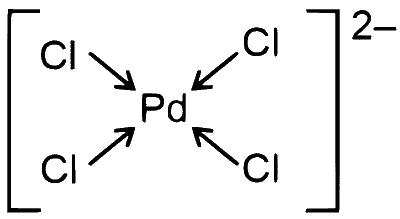
$\left[\mathrm{PdCl}_4\right]^{2-} \Rightarrow \mathrm{Pd}^{2+}, \mathrm{Cl}^{-}$behaves as $\mathrm{SFL}$
$\mathrm{Pd}^{2+} \Rightarrow 4 d^8 \Rightarrow d s p^2 \Rightarrow$ square planer
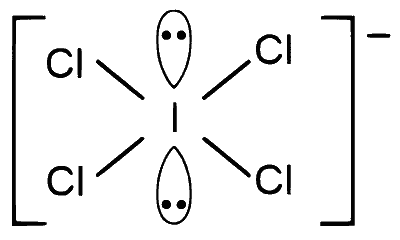
$$ \begin{array}{r} \mathrm{ICl}_4^{\ominus}\Rightarrow 4 \mathrm{bp}+2 \mathrm{lp} \\\\ Hybridisation = s p^3 d^2 \end{array} $$
Geometry = square planer
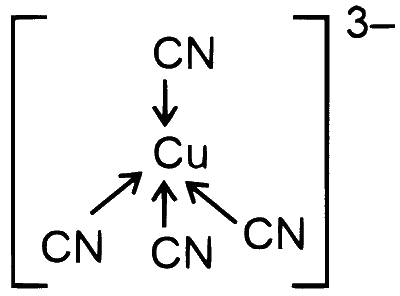
$\begin{aligned} {\left.[\mathrm{Cu}(\mathrm{CN}))_4\right]^{3-} } & \Rightarrow \mathrm{Cu}^{+1} \Rightarrow 3 d^{10} \Rightarrow s p^3\end{aligned}$
Geometry = Tetrahedral
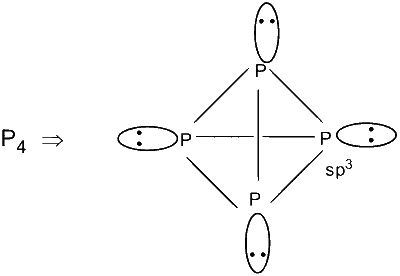
$P_4$ is tetrahedral
Comments (0)


