JEE Advance - Chemistry (2024 - Paper 2 Online - No. 10)
For a double strand DNA, one strand is given below:
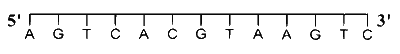
The amount of energy required to split the double strand DNA into two single strands is _______ kcal $\operatorname{mol}^{-1}$.
[Given: Average energy per H-bond for A-T base pair $=1.0 ~\mathrm{kcal}~ \mathrm{mol}^{-1}$, G-C base pair $=1.5 ~\mathrm{kcal}$ $\mathrm{mol}^{-1}$, and A-U base pair $=1.25 ~\mathrm{kcal} ~\mathrm{mol}^{-1}$. Ignore electrostatic repulsion between the phosphate groups.]
Explanation
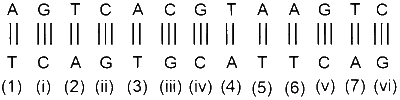
$\mathrm{A}=\mathrm{T} \quad \Rightarrow 2 \mathrm{H}$-bond
$\mathrm{G} \equiv \mathrm{C} \quad \Rightarrow 3 \mathrm{H}$-bond
Number of $\mathrm{A}=\mathrm{T}$ pair $=7$
Number of $\mathrm{G} \equiv \mathrm{C}$ pair $=6$
Number of $\mathrm{H}$-bond involve in $\mathrm{A}=\mathrm{T}=7 \times 2=14$
Number of $\mathrm{H}$-bond involve in $\mathrm{G} \equiv \mathrm{C}=6 \times 3=18$
Energy required for $\mathrm{A}=\mathrm{T}=14 \times 1=14$
Energy required for $\mathrm{G} \equiv \mathrm{C}=18 \times 1.5=27$
Total energy required $14+27=41$
Comments (0)


