JEE Advance - Chemistry (2023 - Paper 2 Online - No. 5)
The complex(es), which can exhibit the type of isomerism shown by $\left[\operatorname{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Br}_2\right]$, is(are) :
[en $=\mathrm{H}_2 \mathrm{NCH}_2 \mathrm{CH}_2 \mathrm{NH}_2$ ]
[en $=\mathrm{H}_2 \mathrm{NCH}_2 \mathrm{CH}_2 \mathrm{NH}_2$ ]
$\left[\mathrm{Pt}(\mathrm{en})(\mathrm{SCN})_2\right]$
$\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]$
$\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_4\right]$
$\left[\mathrm{Cr}(\mathrm{en})_2\left(\mathrm{H}_2 \mathrm{O}\right)\left(\mathrm{SO}_4\right)\right]^{+}$
Explanation
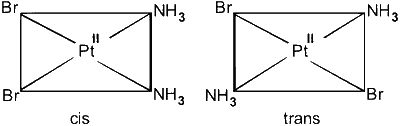
$\left[\operatorname{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Br}_2\right]$
Hybridisation : dsp${ }^2$, geometry : square planar
(A) $\left[\mathrm{Pt}(\mathrm{en})(\mathrm{SCN})_2\right]$ : square planar, cis-trans not possible
(B) $\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]$ : tetrahedral, cis-trans not possible
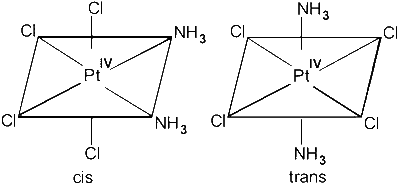
(C) $\left[\operatorname{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_4\right]$ : octahedral, cis-trans possible
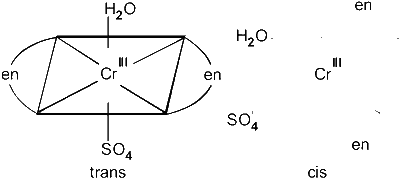
$$ \text { (D) }\left[\mathrm{Cr}(\mathrm{en})_2\left(\mathrm{H}_2 \mathrm{O}\right) \mathrm{SO}_4\right]^{+} \text {: Octahedral } $$
Hybridisation : dsp${ }^2$, geometry : square planar

(A) $\left[\mathrm{Pt}(\mathrm{en})(\mathrm{SCN})_2\right]$ : square planar, cis-trans not possible
(B) $\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]$ : tetrahedral, cis-trans not possible
(C) $\left[\operatorname{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_4\right]$ : octahedral, cis-trans possible

$$ \text { (D) }\left[\mathrm{Cr}(\mathrm{en})_2\left(\mathrm{H}_2 \mathrm{O}\right) \mathrm{SO}_4\right]^{+} \text {: Octahedral } $$

Comments (0)


