JEE Advance - Chemistry (2023 - Paper 2 Online - No. 4)
A disaccharide $\mathbf{X}$ cannot be oxidised by bromine water. The acid hydrolysis of $\mathbf{X}$ leads to a laevorotatory solution. The disaccharide $\mathbf{X}$ is :
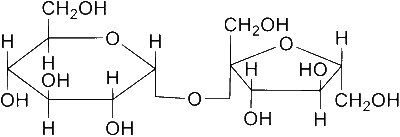



Explanation
A and D cannot be oxidised by bromine water as they do not have hemiacetal linkage.
The acid hydrolysis of A leads to a laevorotatory solution.
A is sucrose which is dextrorotatory, on acid hydrolysis gives mixture of α-D-glucose and β-D-fructose, the mixture is laevorotatory
The acid hydrolysis of A leads to a laevorotatory solution.
A is sucrose which is dextrorotatory, on acid hydrolysis gives mixture of α-D-glucose and β-D-fructose, the mixture is laevorotatory
Comments (0)


