JEE Advance - Chemistry (2023 - Paper 2 Online - No. 17)
The total number of carbon atoms and heteroatoms present in one molecule of $\mathbf{S}$ is _________.
[Use : Molar mass (in $\mathrm{g} \mathrm{mol}^{-1}$ ): $\mathrm{H}=1, \mathrm{C}=12, \mathrm{~N}=14, \mathrm{O}=16, \mathrm{Br}=80, \mathrm{Cl}=35.5$
Atoms other than $\mathrm{C}$ and $\mathrm{H}$ are considered as heteroatoms]
[Use : Molar mass (in $\mathrm{g} \mathrm{mol}^{-1}$ ): $\mathrm{H}=1, \mathrm{C}=12, \mathrm{~N}=14, \mathrm{O}=16, \mathrm{Br}=80, \mathrm{Cl}=35.5$
Atoms other than $\mathrm{C}$ and $\mathrm{H}$ are considered as heteroatoms]
Answer
51
Explanation
Compound S is
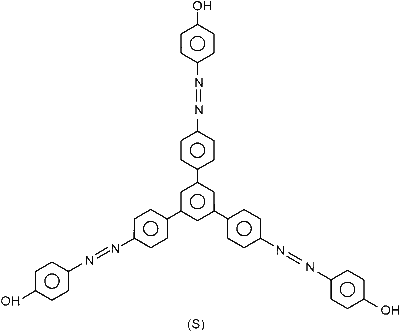
Number of Carbon atoms + Number of Heteroatoms $=51$

Number of Carbon atoms + Number of Heteroatoms $=51$
Comments (0)


